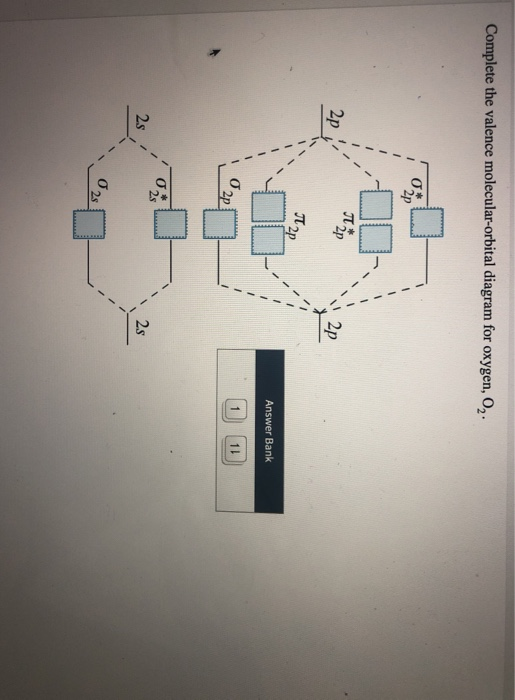
35 complete this valence molecular-orbital diagram for oxygen o2
Chemistry questions and answers. (a) Complete this molecular-orbital energy diagram for oxygen molecule (O2) by filling all the valence electrons in the molecular orbitals (b) please write the name of each molecular orbitals in energy diagram (e.g., o, etc.) (c) Which molecular orbital is highest occupied molecular orbital (HOMO)? (d) Which ... In order to draw oxygen's molecular orbital diagram, you need to start by taking a look at what atomic orbitals you have for an oxygen atom, #"O"#.. As you know, oxygen is located in period 2, group 16 of the periodic table and has an atomic number equal to #8#.This means that the electron configuration of a neutral oxygen atom must account for #8# electrons.
Complete this valence molecular orbital diagram for oxygen o2. Click the blue boxes to add electrons as needed. Complete these structures by adding bonds and lone pairs as necessary. This video shows the construction of a molecular orbital mo diagram for the diatomic molecule o2 using the valence electrons of each oxygen.
Complete this valence molecular-orbital diagram for oxygen o2
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
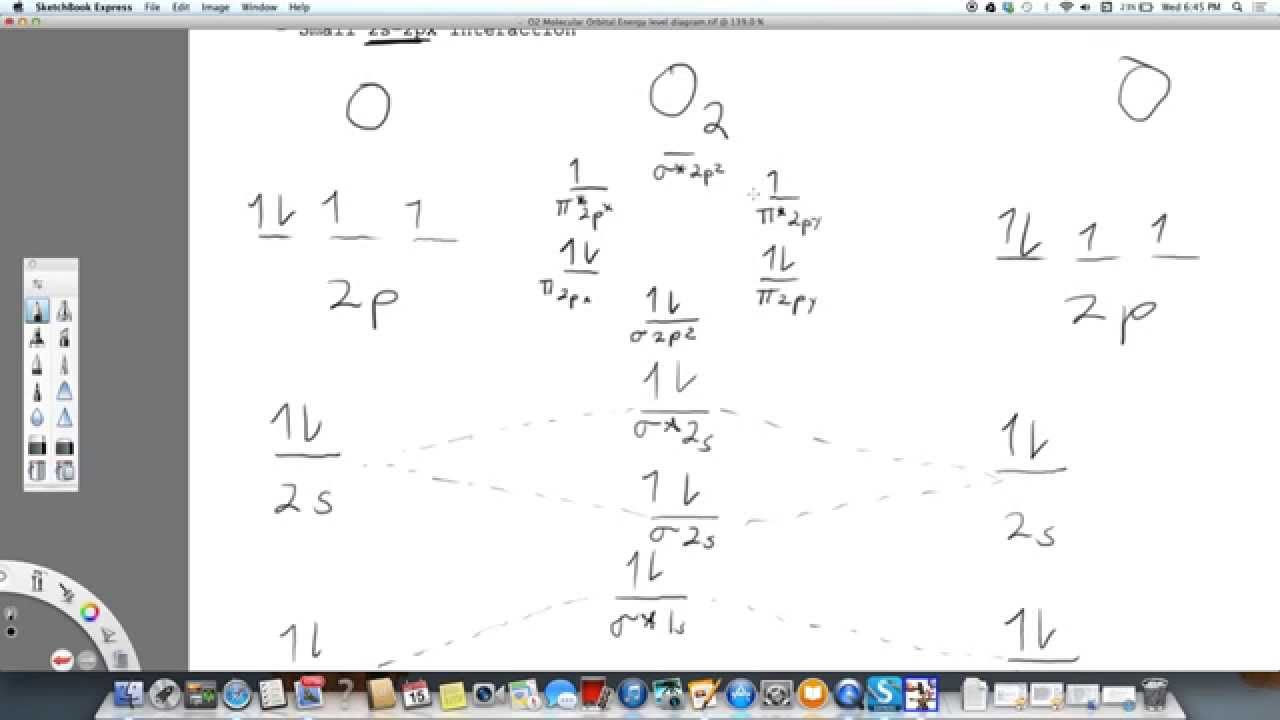
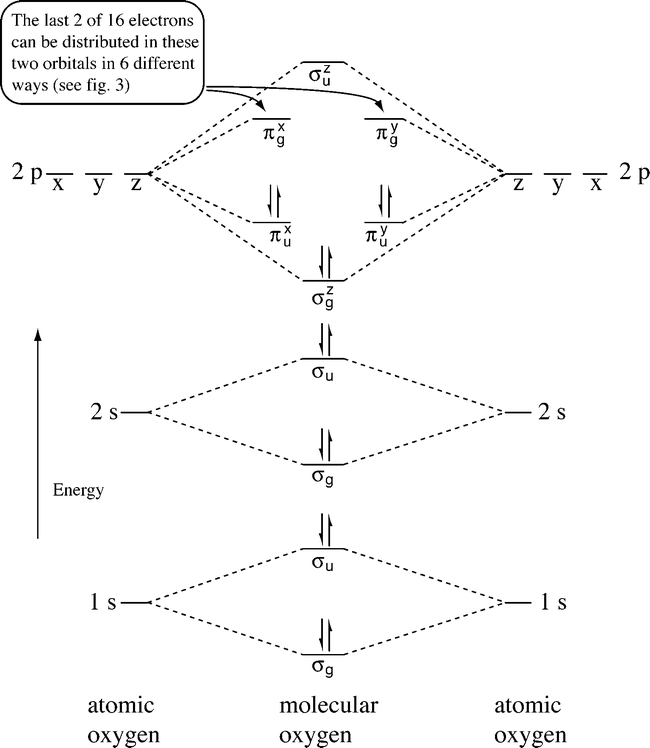
Complete this valence molecular-orbital diagram for oxygen o2. Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. The last two electrons in p 2 p x ∙ and p 2 p y ∙ orbitals will remain unpaired. Therefore, oxygen molecule has paramagnetic character due to the presence of two unpaired electrons. Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... FREE Answer to Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on molecular orbital diagram. A molecular orbital diagram is used to explain ...
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.


















0 Response to "35 complete this valence molecular-orbital diagram for oxygen o2"
Post a Comment