36 lewis dot diagram for iodine
1 answerWhat should the electron dot diagram for iodine look like? Chemistry Drawing Lewis Structures. 1 Answer. Jahan Psyche. Oct 24, 2015. peoi.org. Intermolecular forces practice worksheet answers
Iodine is a naturally occurring element found in sea water and in certain rocks and sediments. There are non radioactive and radioactive forms of iodine. Iodine is used as a disinfectant for cleaning surfaces and storage containers and is used in skin soaps and bandages, and for purifying water. Iodine is also added to some table salt to ensure that all people in the United States have enough ...
Lewis dot diagram for iodine
Shape of xef4 Draw the Lewis (electron dot) ... Reaction kinetics can be investigated using the iodine clock reaction. ... Include in the diagram the direction of the electron flow, the polarity of electrodes and state the half-equations for the product formed at each electrode. [5] a.i. 1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ...
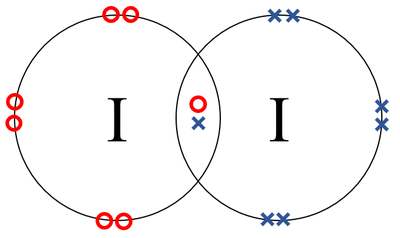
Lewis dot diagram for iodine. Nov 04, 2021 · Ch3ch2nh2 lewis dot structure. H H Apago PDFH HEnhancer A A to write a Lewis structure for ethane H C C H HH or HOCOCOH A A H H PROBLEM 1. (c) Methyl fluoride (CH3F) (a) Propane (C3H8) (b) Methanol (CH4O) (d) Ethyl fluoride (C2H5F) C H A P T E R1The Basics BONDING AND MOLECULAR STRUCTUREOrganic chemistry plays a role in all aspects of our liv The structure of ATP has an ordered carbon compound ... 28 Apr 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ... 1:36A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the ...4 May 2013 · Uploaded by Wayne Breslyn Iodine is a diatomic molecule and contains only two iodine atoms. Lewis structure of iodine molecule contains only one I-I bond and each iodine atom has ...
Nov 11, 2021 · H2co resonance structures The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation (loss of electrons) of an atom in a chemical compound.Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. For identified element identify the ground state electron configuration, orbital diagram, Lewis dot diagram, and number of valence. (b) Hydrogen The correct set of quantum numbers for the unpaired electron of a chlorine atom is 3, 1, 1, ± 2 1 . Lewis diagram for the ether c2h5oc2h5
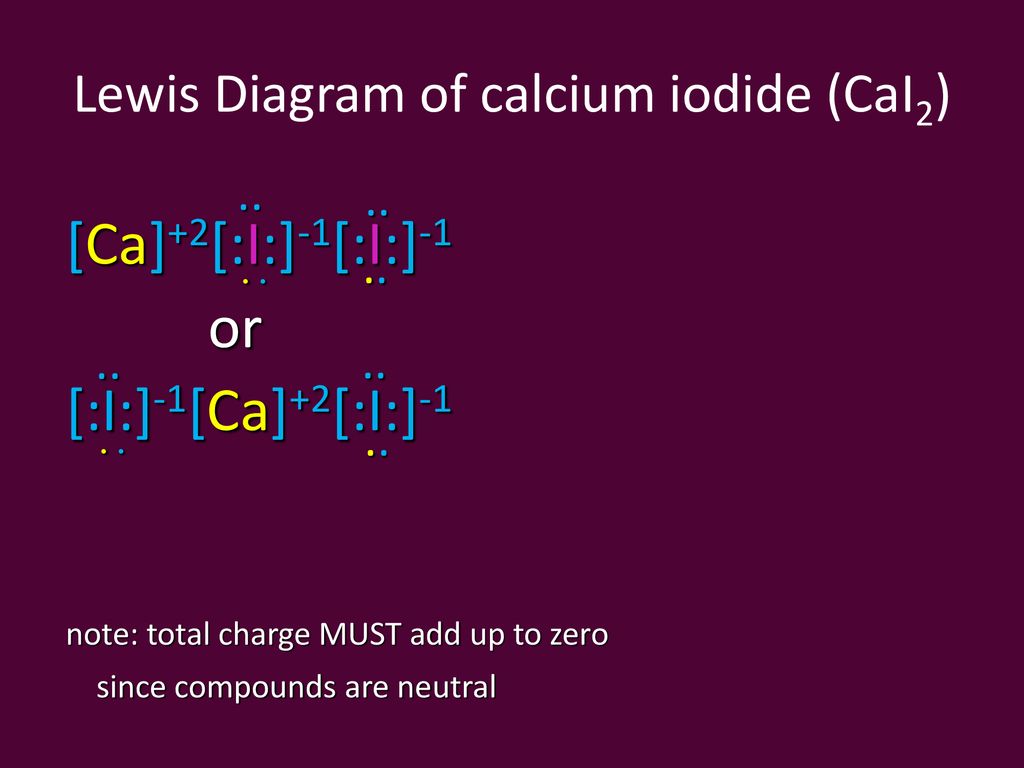
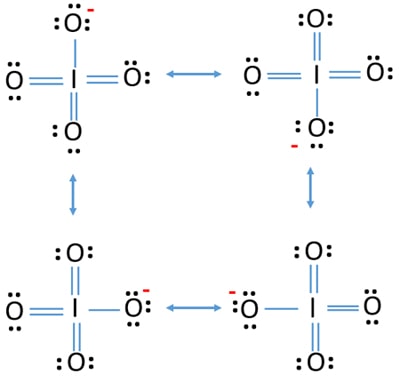
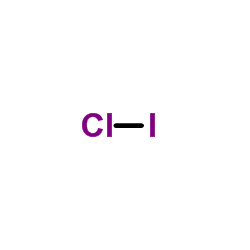
(It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for calcium is simply. A Lewis structure of calcium is shown ... Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...25 Oct 2016 · Uploaded by Wayne Breslyn A Lewis Structure (electron dot diagram) shows us how the bonding and non-bonding valence electrons are arranged around the atoms. A structural formula shows us how the covalent bonds are arranged around the atoms in a molecule. A condensed structural formula is a more compact way of drawing the structural formula of a molecule. Iodine dichloride (ICl2-) lewis dot structure, ... the least electronegative atom takes the middle position in the lewis diagram. In the case of the ICl2- molecule, both iodine and chlorine atoms belong to the same periodic group but “the electronegativity of an element decrease as we down the group”.
Some sources indicate the Lewis base with a pair of dots (the explicit electrons being donated), which allows consistent representation of the transition from the base itself to the complex with the acid: Me 3 B + :NH 3 → Me 3 B:NH 3. A center dot may also be used to represent a Lewis adduct, such as Me 3 B•NH 3.
Nov 15, 2021 · Lewis Structure. To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure: Step 1
Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ...
F1-O-2. Therefore, the s subshell can hold 2 electrons; the p can hold 6; the d can hold 10; and the f can hold 14. Complete the Lewis dot diagram and. For the following atoms and ions write the full electron configuration and the short hand configuration carbon full 1s 2 2s 2 2p 6 short hand hev2s 2 2p 6 bromine full 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

Yodium Pentafluoride Struktur Lewis Iodine Heptafluoride Arsenic Pentafluoride Lainnya Sudut Teks Lain Lain Png Pngwing
1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ...
Draw the Lewis (electron dot) ... Reaction kinetics can be investigated using the iodine clock reaction. ... Include in the diagram the direction of the electron flow, the polarity of electrodes and state the half-equations for the product formed at each electrode. [5] a.i.
Shape of xef4
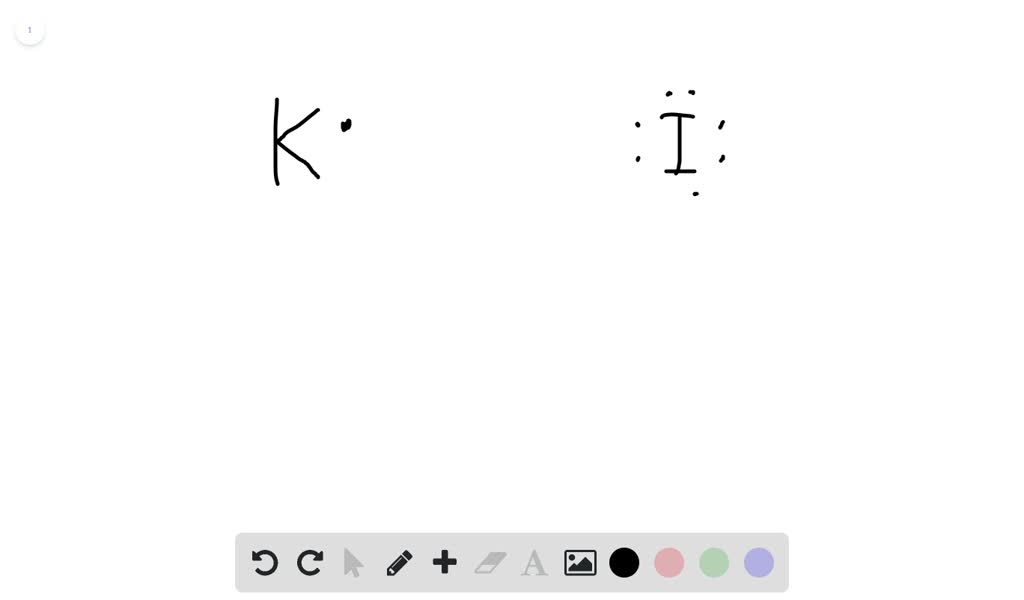
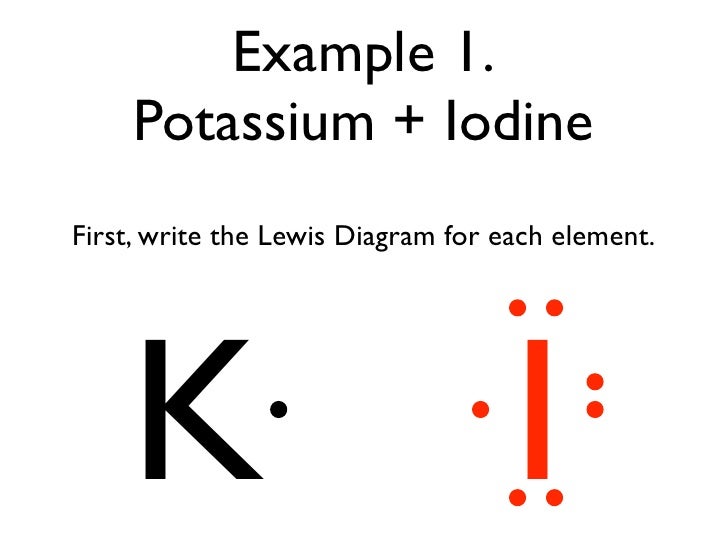
Solved Using Electron Dot Structures Diagram The Formation Of An Ionic Bond Between Potassium And Iodine
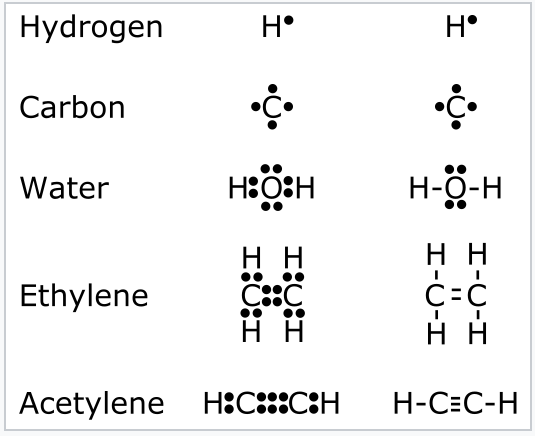
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts


















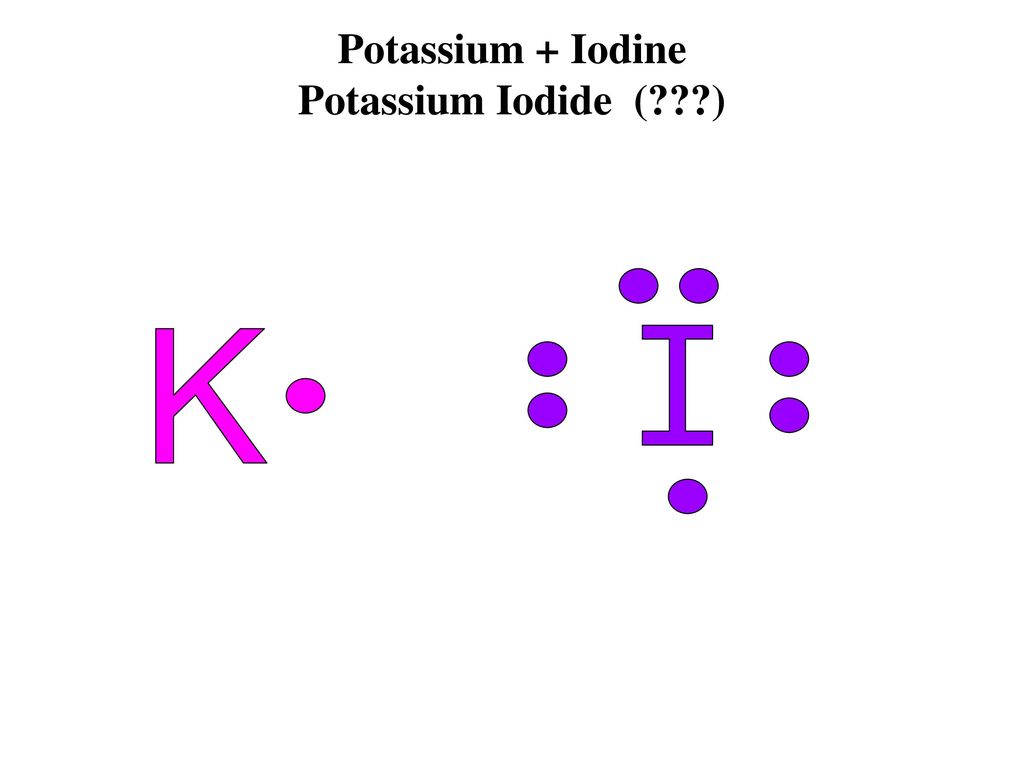
0 Response to "36 lewis dot diagram for iodine"
Post a Comment