36 molecular orbital diagram for b2
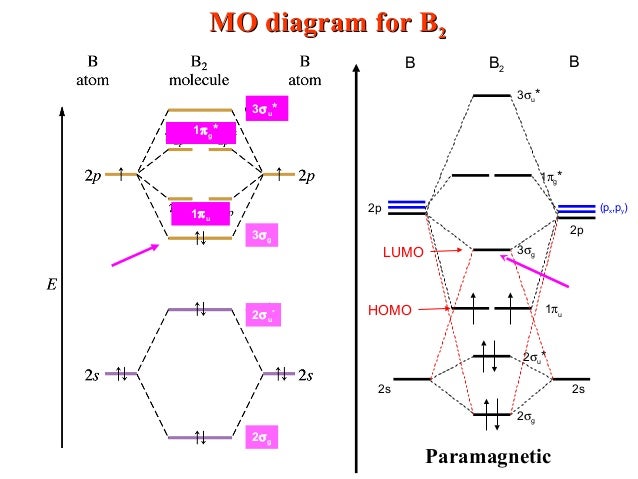
Consider the molecule B2 (explored above in question #4). What is the magnetism and number of unpaired electrons in B2? paramagnetic, 2 . According to molecular orbital theory, which of the following is NOT predicted to exist? He2 When the bond order is equal to 0, a molecule is predicted to not exist. bond order = [(# of bonding elections# of anti bonding electrons) / 2] For He2, the number ... ⇒ A homo-nuclear diatomic molecular orbital in which the same atoms combine together. example- N2, O2, B2, etc. ⇒ A hetero-nuclear diatomic molecular orbital in which different atoms combine together. example- CN, HF, NO, etc. Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the Molecular orbital diagram of CN-we can ...
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate ...
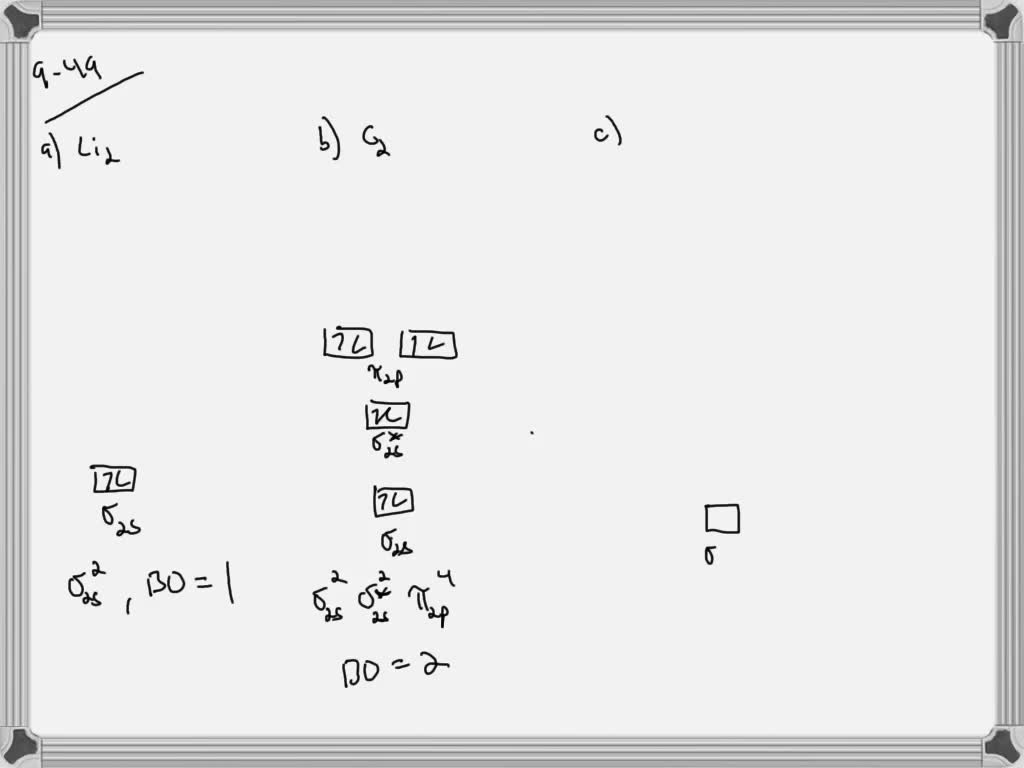
Molecular orbital diagram for b2
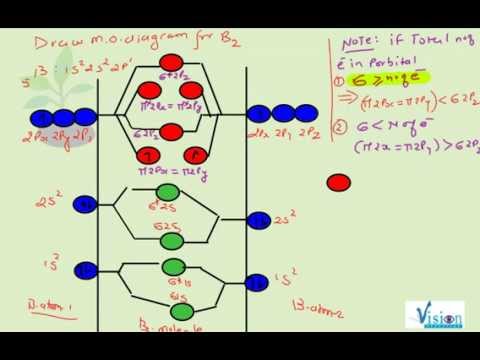
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order ...2 Jun 2021 · Uploaded by Principia The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for ${{\text{B}}_{\text{2} ... B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 ... Rating: 4,4 · 740 votes · Free · Android · Educational
Molecular orbital diagram for b2. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is formed as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both ... Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired ... B2 molecular orbital diagram. Since bond order is zero be 2 molecule does not exist. This was on a quiz and i somehow got the bond order and the lumo indicated wrong. I also calculated the bond order of this molecule to be 32. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. The resulting diagram looks (in approximation) like this: If you now fill the three valence electrons of each boron into the diagram according to Hund's rule, you will see that each π orbital will get one electron. This results in a triplet ground state. The finished valence molecular orbital diagram is pictured below.
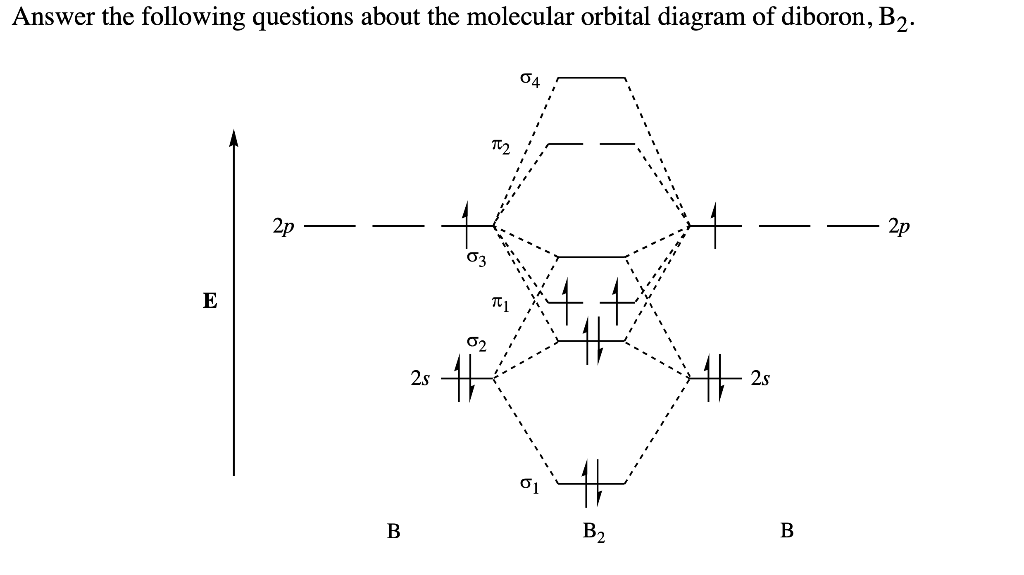
This problem has been solved! See the answer. The Molecular Organization diagram below is appropriate for B2. Based on this diagram, a)draw the molecular orbital filling diagram for B2. b) what is the bond order of B2. c) is B2 diamagnetic or paramagnetic. Show transcribed image text. As comparison, our previous work about B2 and B3, the analogous to DTBT and DF ... The first oxidation wave of each HTM is assigned to an electron extraction from highest occupied molecular orbital (HOMO), which was calculated to be −5.25, −5.28 and −5.30 eV for DTBT, SF-DTBT and DF-DTBT, respectively. It's found that the electron withdrawing of substituent lowers the HOMO, which ... Academia.edu is a platform for academics to share research papers. The word comet derives from the Old English cometa from the Latin comēta or comētēs.That, in turn, is a romanization of the Greek κομήτης 'wearing long hair', and the Oxford English Dictionary notes that the term (ἀστὴρ) κομήτης already meant 'long-haired star, comet' in Greek. Κομήτης was derived from κομᾶν (koman) 'to wear the hair long', which was itself ...
10:50From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of ...24 Jun 2020 · Uploaded by Edmerls Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... 11.11.2016 · So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = .5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. I always thought bond order corresponded to the number of bonds. That is, molecule held together by a single bond … Academia.edu is a platform for academics to share research papers.

Solved Draw The Molecular Orbital Diagrams For B2 C2 And N2 Please Use It To Predict The Bond Order For B2 B2 B2 C2 C2 C2 N2 N2 And N2 Which
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond ...30 May 2021 · Uploaded by Principia

3 Which Of The Following Species Have Both O And It Bond According To Molecular Orbital Theory 1 Nz 2 B2 3 Cz Canly Tband 4 All Of These
6:25This video discusses how to draw the molecular orbital (MO) diagram for the B2(+) molecule. The bond ...4 Jun 2021 · Uploaded by Principia
A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, H 2), and the formation of H II regions.This is in contrast to other areas of the interstellar medium that contain predominantly ionized gas.
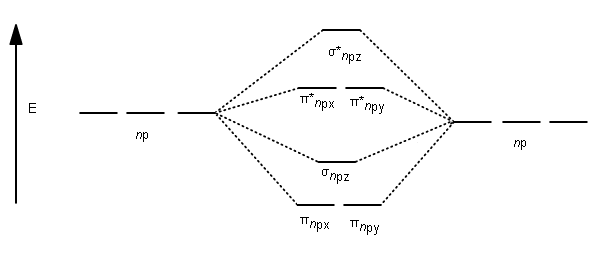
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
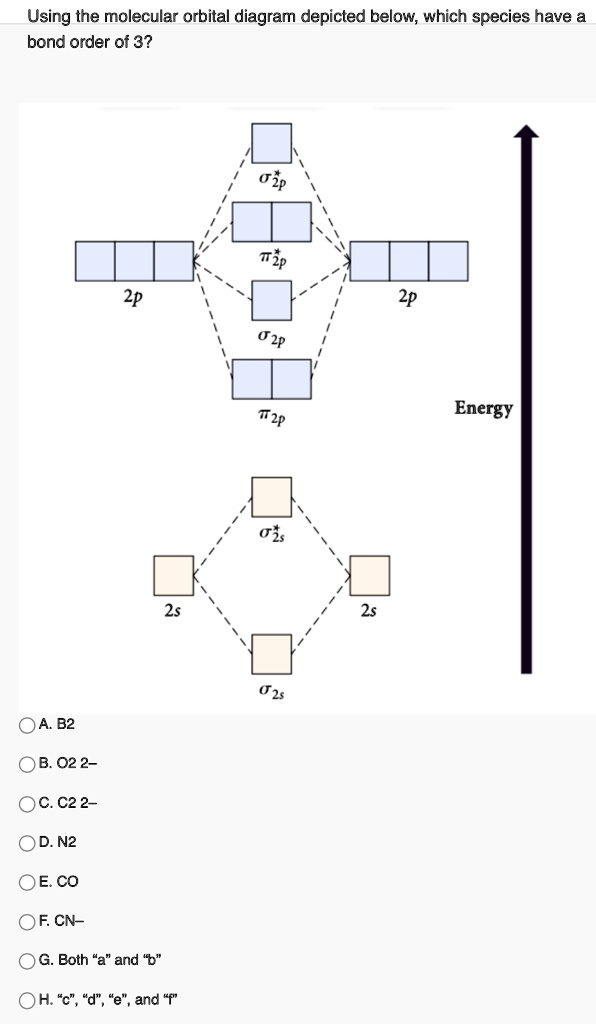
Solved Using The Molecular Orbital Diagram Depicted Below Which Species Have Bond Order Of 3 2p 2p 02p 72p Energy 2s Oa B2 B 02 2 C C22 D N2 Oeco Of Cn G
Molecular orbital diagram for b2. This interaction introduces an element of s p mixing or hybridization into the molecular orbital theory. When p s mixing is allowed the energies of the σ2p and π2p orbitals are reversed. The two electrons from the b 2p orbitals now occupy separate degenerate π2p molecular orbitals and thus have parallel spins.
14:24This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the ...26 Mar 2014 · Uploaded by Diego Troya

Part A By Drawing Molecular Orbital Diagrams For B2 C2 N2 O2 And F2 Predict Which Of These Brainly Com
Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *).
7:14The bond order of B2, C2, and N2 are 1, 2, and 3, respectively. B2 has two unpaired electrons with the same ...20 Jun 2019 · Uploaded by Physical Chemistry Tutorial
Molecular orbital diagram of b2. The next two would fill the 1 sigma e antibonding orbital. I can draw be2 but not this. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. The electronic configuration of b atom z 5 is. It is diamagnetic due to the absence of any unpaired electron.
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 ... Rating: 4,4 · 740 votes · Free · Android · Educational
The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for ${{\text{B}}_{\text{2} ...
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order ...2 Jun 2021 · Uploaded by Principia

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond
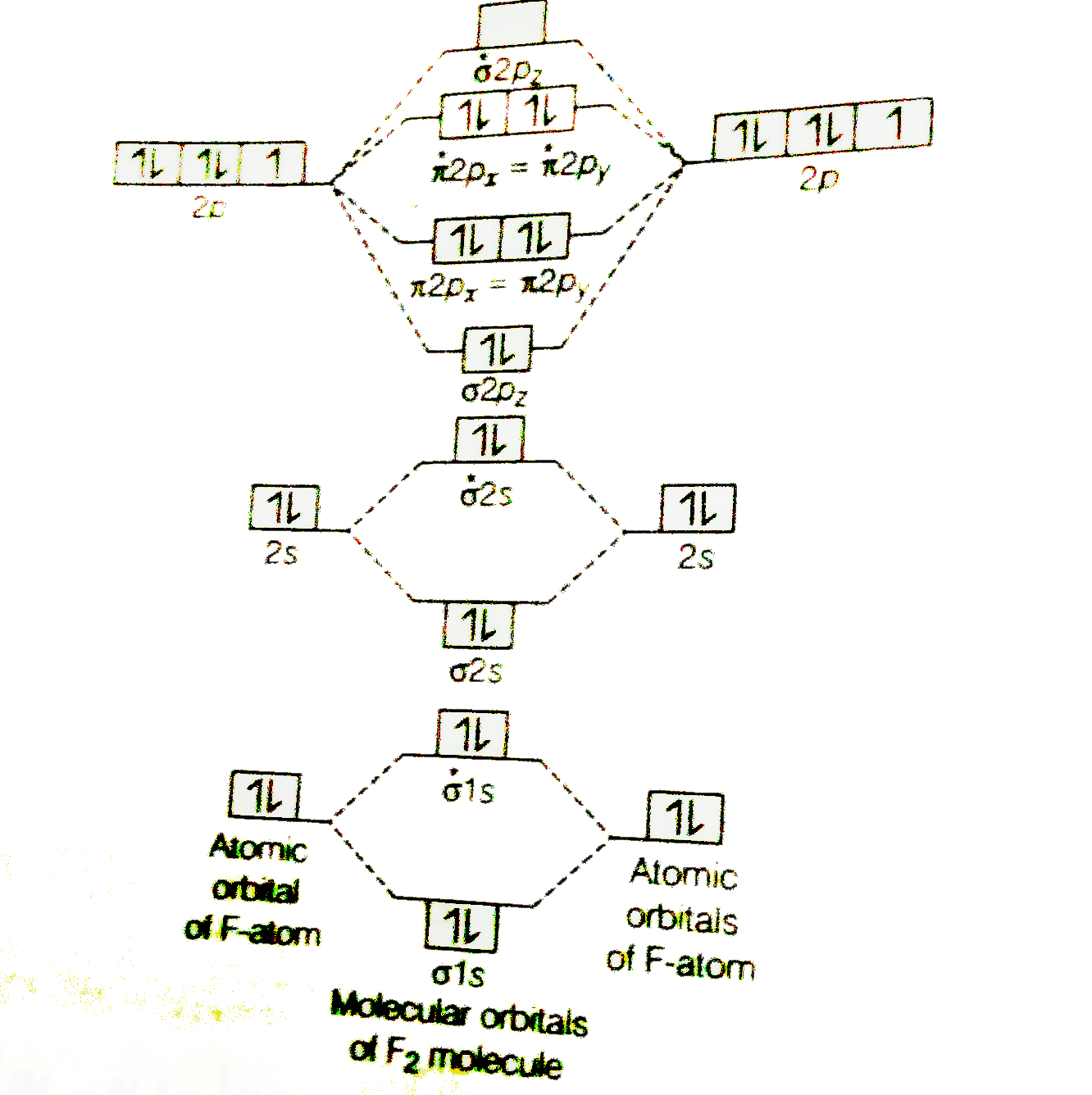
Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond

Write Molecular Orbital Configuration Of C2 Predict Magnetic Behaviour And Calculate Its Bond Order H7ch14qq Chemistry Topperlearning Com

Bonding In B2 And B2 Insights From Full Configuration Interaction And Valence Bond Studies Sciencedirect

4 On The Basis Of Molecular Orbitals And Molecular Orbital Diagrams Predict Which Molecule In Each Homeworklib

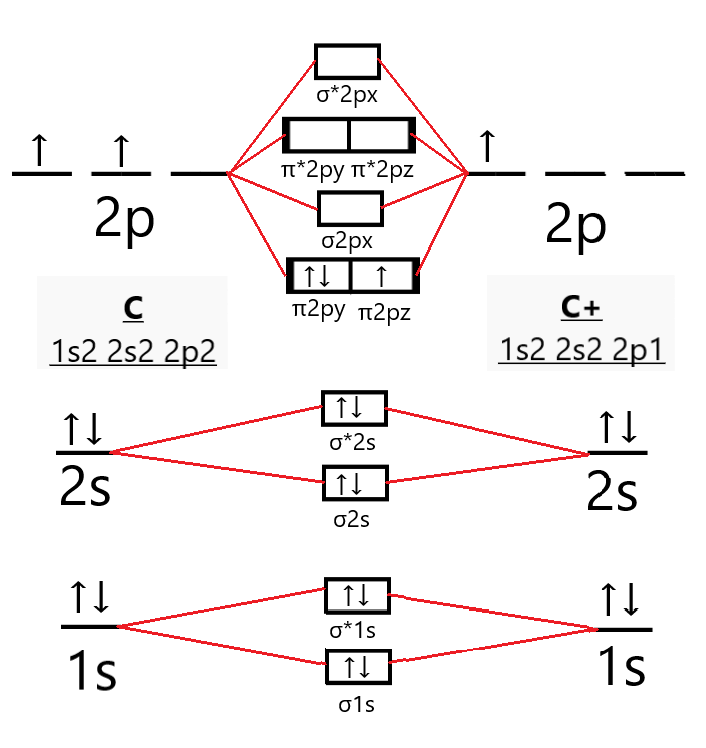












0 Response to "36 molecular orbital diagram for b2"
Post a Comment