38 magnesium electron dot diagram
Answered on 22nd Feb, 2021. (a) Magnesium has Atomic Number = 12. Its electronic configuration is 2,8,2. Chlorine has Atomic Number = 17. Its electronic configuration is 2,8,7. (b) The electron-dot structures of magnesium and chloride are given below: (c) Magnesium Chloride is formed by the transfer of 2 electrons from the outer shell of the ... Jul 03, 2019 · Lewis Electron Dot Diagrams . Lewis electron dot diagrams may be drawn to help account for the electrons participating in a chemical bond between elements. A Lewis diagram counts the valence electrons. Electrons shared in a covalent bond are counted twice. For the octet rule, there should be eight electrons accounted for around each atom.
Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
Magnesium electron dot diagram
Answer : The Lewis-dot structure of phosphorus trihydride is shown below. Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The valence electrons are represented by 'dot'. The given molecule is, phosphorus trihydride. As we know that phosphorous has '5 ... Electron dot structural diagram : Thus Calcium donates 2 electrons to oxygen to become Ca 2+ and Oxygen accepts 2 electrons to become O 2-. Strong electrostatic forces of attraction develop between the two oppositely charged ions and this leads to formation of Calcium oxide molecule. c. Magnesium chloride molecule June 4th, 2018 - Create a Lewis dot structure for an near the end of the bonding unit to reinforce the differences between ionic and covalent bonding Strontium Phosphide''magnesium chloride lewis dot structure document at Agrisa what is lewis structure for magnesium phosphide?. Sell Used Ac Units, Magnesium fluoride doesn't have a Lewis structure.
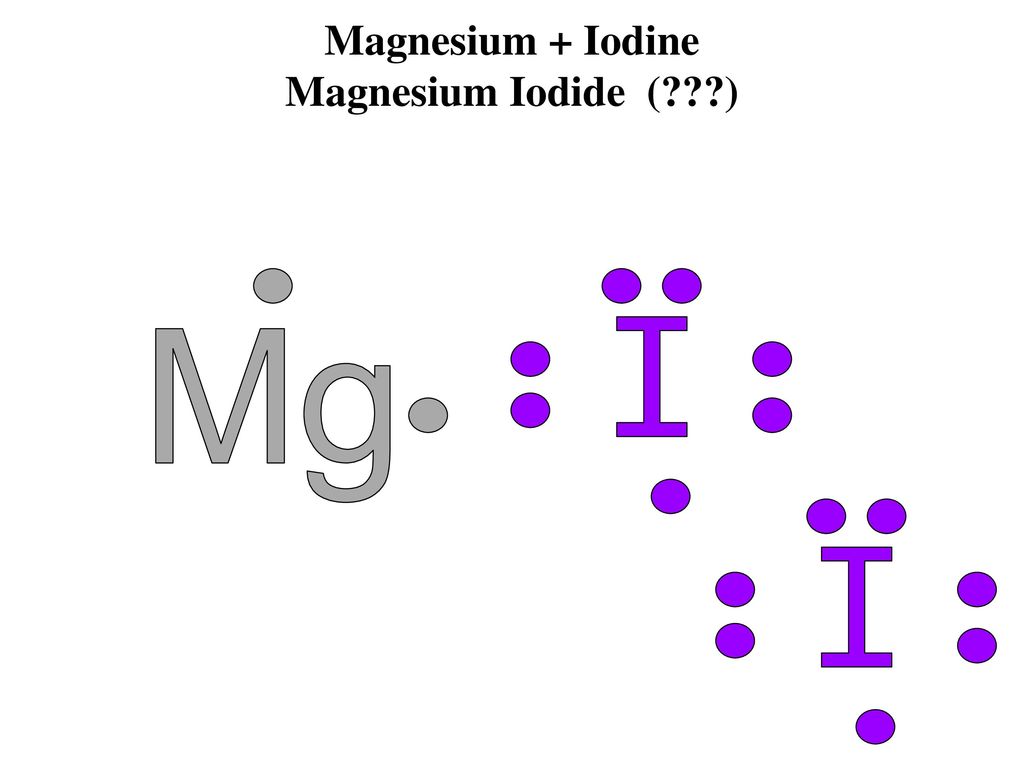
Magnesium electron dot diagram. Draw The Lewis Structure Of Mgbr2 Magnesium Bromide Youtube . Magnesium has an electronic configuration of 282. Magnesium bromide electron configuration. 47 1959 x 2 of 4 Describe the electron transfers that occur in the formation of magnesium bromide from elemental magnesium and elemental bromine. Hence it has a charge of 2 Mg2. The dot-cross diagram is an essential part of explaining how covalent bonding works. A dot-cross diagram consists of nothing but circles, dots, and crosses. The dot represents electron(s) from one atom, while the cross represents electron(s) from the other atom. When one pair of the electron is shared, it means one covalent bond is formed. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). Golden Retrievers Waco Tx, The central atom of this molecule is carbon. Nds To Cia Converter, is the Lewis diagram for the formation of magnesium chloride. Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot …
MgCl2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. MgCl2 is an ionic halide salt consisting of magnesium and chlorine elements. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. In its anhydrous form, it consists of about 25.5% Mg by mass and has a molar mass of about 95.211 g/mol. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Apr 6, 2017 — Note: Magnesium is in Group 2 (sometimes called Group II or 2A). Since it is in Group 2 it will have 2 valence electrons. When you draw the Lewis structure for ...3 answers · 3 votes: draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:)What is the Lewis structure for magnesium bromide?1 answerJun 14, 2016What is the Lewis Dot Structure for Mg3N2?3 answersOct 22, 2018More results from www.quora.com This will form MgCl2. There are two ions involved in the formation of magnesium chloride, two electrons are removed from the magnesium ion, then the cation will ...1 answer · Top answer: Hint: To draw the electron dot structure, we have to first write the electronic configuration of the element then find the valence shell and find the ...
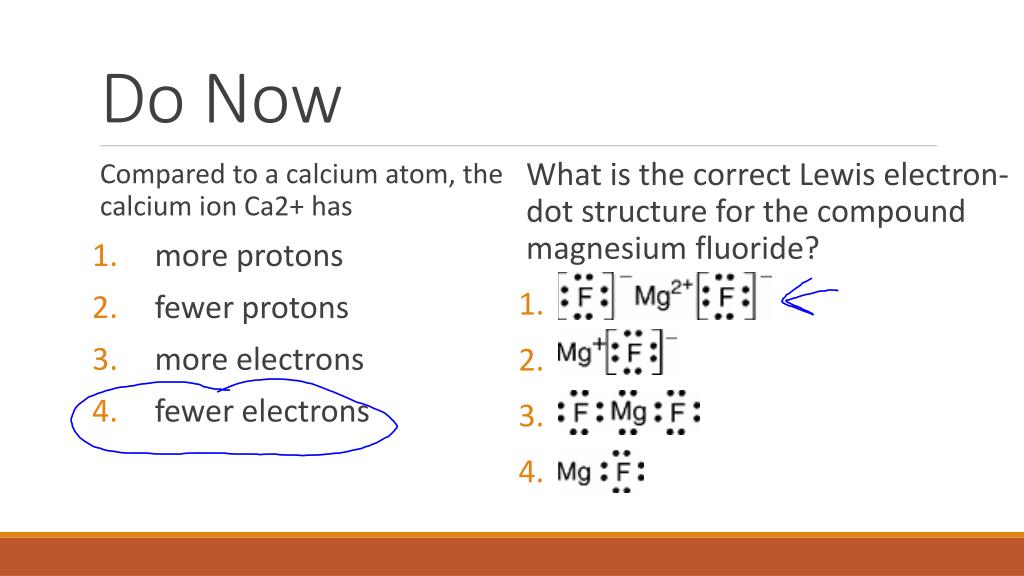
Valence Electrons and Electron-Dot Symbols ... The electron-dot symbol for Mg shows two valence electrons as single dots on the sides of the symbol Mg. Does the model correctly represent the electron dot diagram of magnesium? Why or why not? Select two options. M g with 1 dot above and 1 right. Yes, because the chemical symbol of magnesium is Mg. No, because the chemical symbol of magnesium is Mn. Yes, because magnesium has two valence electrons. No, because magnesium has seven valence electrons. Feb 23, 2012 — Vocabulary. core electron; Lewis dot diagram; valence electron; valence shell ... Therefore, magnesium has two valence electrons. Explain with the help of (i) an ionic equation (ii) electron dot structural diagram (iii) atomic or orbital structural diagram for the formation of the follo. Explain with the help of (i) an ionic equation (ii) electron dot structural diagram (iii) atomic or orbital structural diagram for the formation of the follo.
Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds I Methane Sarthaks Econnect Largest Online Education Community
Lithium oxygen neon magnesium iodine boron sulfur carbon phosphorus ii. You should consult the lewis structure rules and a periodic table while doing this exercise. Write the electron dot structure lewis dot structure for covalent compounds or ions. If you are not sure if your structure is correct do a formal charge check.
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
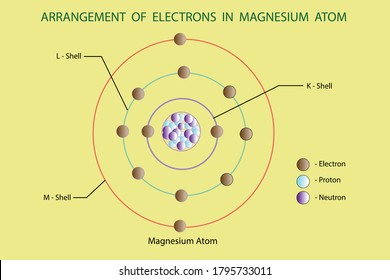
Which is the electron dot structure of magnesium? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
What is the electron dot structure of magnesium? When you write the electron dot structure, all the electrons of the valence shell should be considered, valence shell is the last shell in the electronic configuration, and in both the magnesium and chlorine, the valence shell is 3.
Solved What Is The Lewis Structure Chemical Formula And Word Formula For The Combination Of Magnesium Mg And Sulfur S Course Hero
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
Lewis Structure of Magnesium Fluoride (MgF2) Magnesium fluoride (MgF2) is not a molecular/covalent compound; magnesium has a low electronegativity (it is a metal, after all) and fluorine has a high electronegativity (it is a non-metal, halogen, and has the highest electronegativity of all atoms on the table). So, a transfer of electrons occurs.
Electron Diagram Of Magnesium Electron Dot Structure For Electron Configuration Electrons Magnesium . The Electron Configuration For Chlorine Is 1s2 2s2 2p6 3s2 3p5 Christmas Bulbs Electron Configuration Christmas Ornaments . See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Atomic Structure
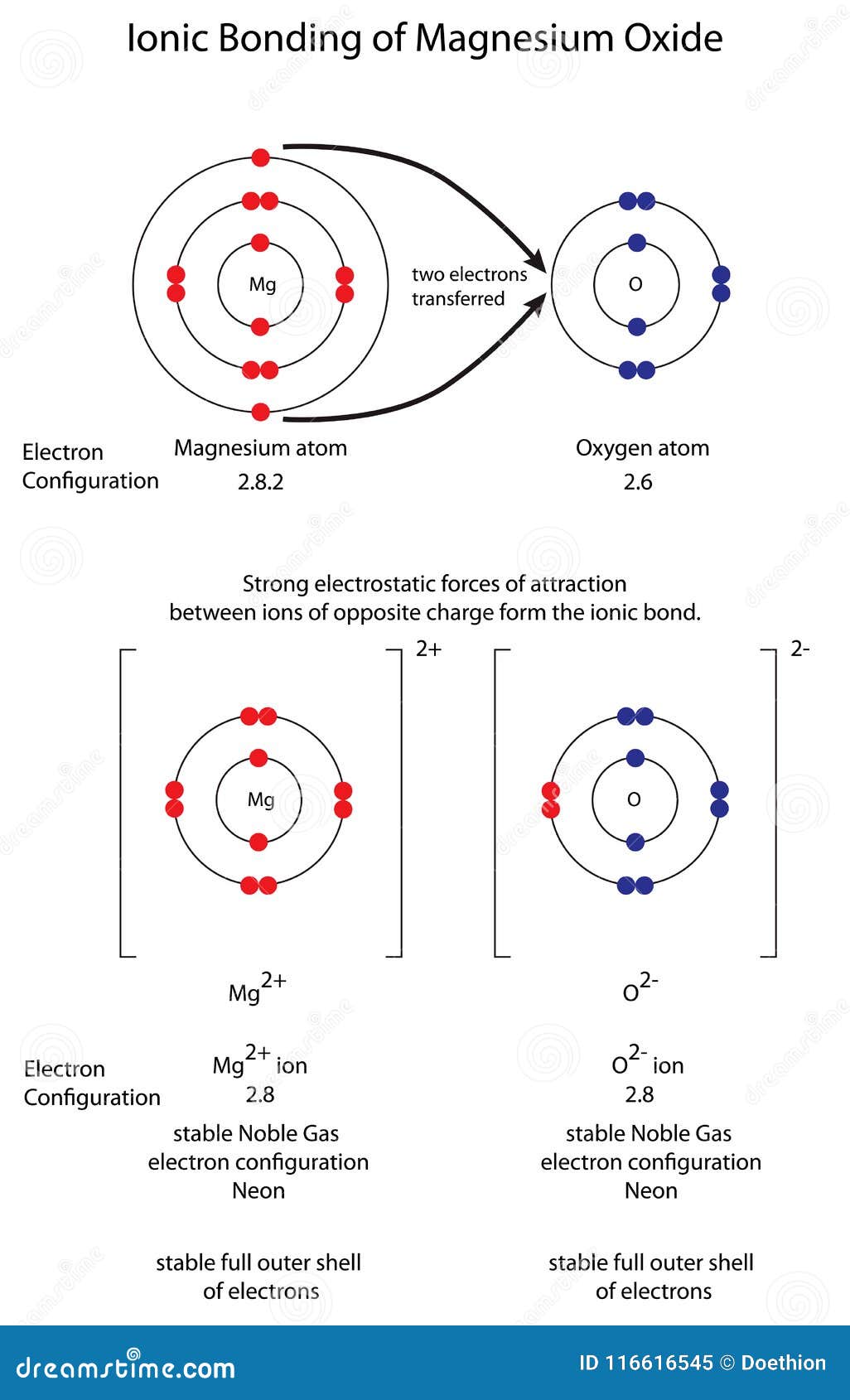
Diagram To Show Ionic Bonding In Magnesium Oxide Mgo Stock Illustration Illustration Of Compound Formation 116616545
Magnesium electron dot diagram. Asked by wiki @ 16/06/2021 in Chemistry viewed by 15 persons. Which diagram is the correct electron dot diagram for magnesium? Ccl4 covalent compound name. Asked by wiki @ 16/06/2021 in Chemistry viewed by 32 persons.
Mar 9, 2020 - Lewis Dot Structure for Magnesium(Mg)||Lewis Structure of Magnesium(Mg)

Lewis Dot Structures Dots Are Arranged Around An Element S Symbol To Indicate The Valence Electrons Reminder Valence Shell Only Has S P Orbitals Maximum Ppt Download
(i) Electron dot diagram of Magnesium: Electron dot diagram of Oxygen: (ii) Formation of MgO: Mg loses 2 electrons and oxygen gains two electrons to complete their octet and form the compound MgO. (iii) The ions present in the compounds are Mg 2+ and O 2-.
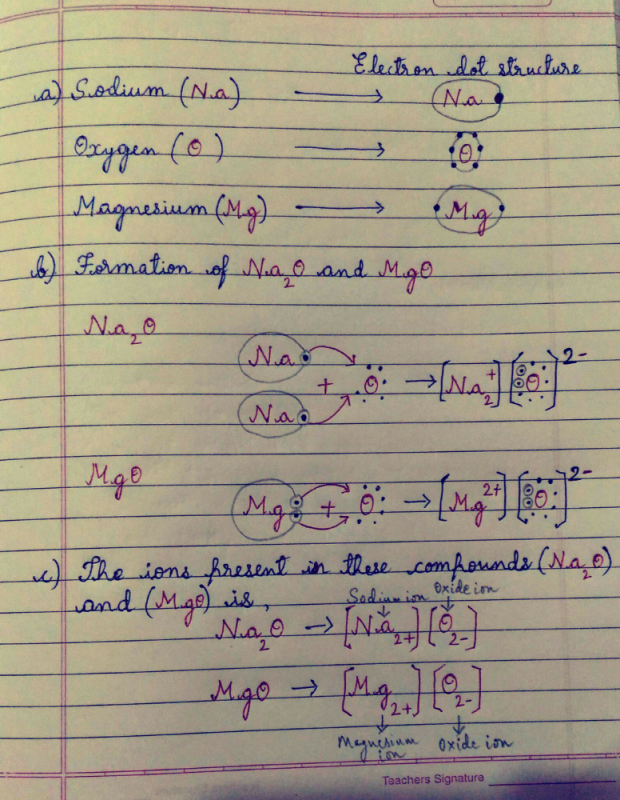
A Write The Electron Dot Structure For Sodium Oxygen Magnesium B Show The Formation Of Na2o And Mgo By The Transfer Of Electron C What Are The Ions Present In These Compounds Edurev
Feb 18, 2019 · Step 4 of creating a Lewis dot structure is choosing a central atom for the other atoms to branch off from in the diagram. The central atom of a molecule is typically the atom with the highest electron valence of the atom with the lowest level of electronegativity.
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. Which is the electron dot structure of ...
Explain the formation of magnesium oxide from magnesium and oxygen? Analyze the electron dot diagram and complete the table. Answer: To attain stability magnesium donates 2 electrons to become magnesium ion (Mg 2 +) and - oxygen become [O 2 -] ion. This type of bonding is ionic bonding.
What is the Lewis dot structure for magnesium?, All the Elements in a Column Have the Same Electron Dot Diagram Element # Valence e- # Valence e- Beryllium (Be) 2 2 Magnesium (Mg) 2 2 Calcium (Ca) 2 2 Furthermore, Is magnesium fluoride ionic or covalent?, Magnesium fluoride is not MgFl, but MgFl2.
For example, the Lewis electron dot diagram for calcium is simply ... a) Mg 2+. b) S 2−. 10. Draw the Lewis electron dot diagram for each ion.
Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw. draw electron dot structure of ethane and butane, Figure 4 demonstrates drawing Lewis electron dot structures for these compounds. give the a electron dot diagram of i magnesium chloride ii nitrogen iii methane ...
The electron configuration for magnesium is shown below. 1s22s22p63s2 Which diagram shows the correct distribution of electrons in the electron shells of a magnesium atom? A. mc025-1.jpg B. mc025-2.jpg C. mc025-3.jpg D. mc025-4.jpg
Give electron dot diagram of the following: (a) Magnesium chloride (b) nitrogen (c) methane asked Sep 6, 2018 in Chemistry by PriyaBharti ( 53.7k points) chemical bonding

Draw The Electron Dot Diagram Of The Following Compounds 1 Naf 2 Mgf2 Hint Atomic No Na 11 F 9 Mg 12
Does the model correctly represent the electron dot diagram of magnesium? Why or why not? Select two options. M g with 1 dot above and 1 right. Yes, because the chemical symbol of magnesium is Mg. No, because the chemical symbol of magnesium is Mn. Yes, because magnesium has two valence electrons. No, because magnesium …
The former, known as a ‘Lewis dot diagram,’ indicates a pair of shared electrons between the atomic symbols, while the latter, known as a ‘Lewis structure,’ uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
How many dots does magnesium have? element. The electron-dot symbol for Mg shows two valence electrons as single dots on the sides of the symbol Mg. What is the Lewis dot structure for beryllium? A beryllium atom, with two valence electrons, would have the electron dot diagram below….Electron Dot Diagrams.
Lewis Structure of MgO, Magnesium Oxide. Magnesium oxide is an ionic compound. Its formula unit (MgO) is made from one magnesium atom, which loses two electrons to become a +2 cation, and one oxygen atom, which gains those two electrons to become a -2 anion. The Lewis Structure can show the transfer of electrons from metal (low ...
Electron configuration for sodium (Na). The electron configuration is: 1s22s22p63s1. · Lewis dot Na.svg · Electron configuration for magnesium (Mg) · Lewis dot Mg.A Simplified Way to Show... · All the Elements in a Column... · Lesson Summary
Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a way that's easy for you to ...
June 4th, 2018 - Create a Lewis dot structure for an near the end of the bonding unit to reinforce the differences between ionic and covalent bonding Strontium Phosphide''magnesium chloride lewis dot structure document at Agrisa what is lewis structure for magnesium phosphide?. Sell Used Ac Units, Magnesium fluoride doesn't have a Lewis structure.
Electron dot structural diagram : Thus Calcium donates 2 electrons to oxygen to become Ca 2+ and Oxygen accepts 2 electrons to become O 2-. Strong electrostatic forces of attraction develop between the two oppositely charged ions and this leads to formation of Calcium oxide molecule. c. Magnesium chloride molecule
Answer : The Lewis-dot structure of phosphorus trihydride is shown below. Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The valence electrons are represented by 'dot'. The given molecule is, phosphorus trihydride. As we know that phosphorous has '5 ...


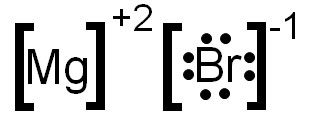



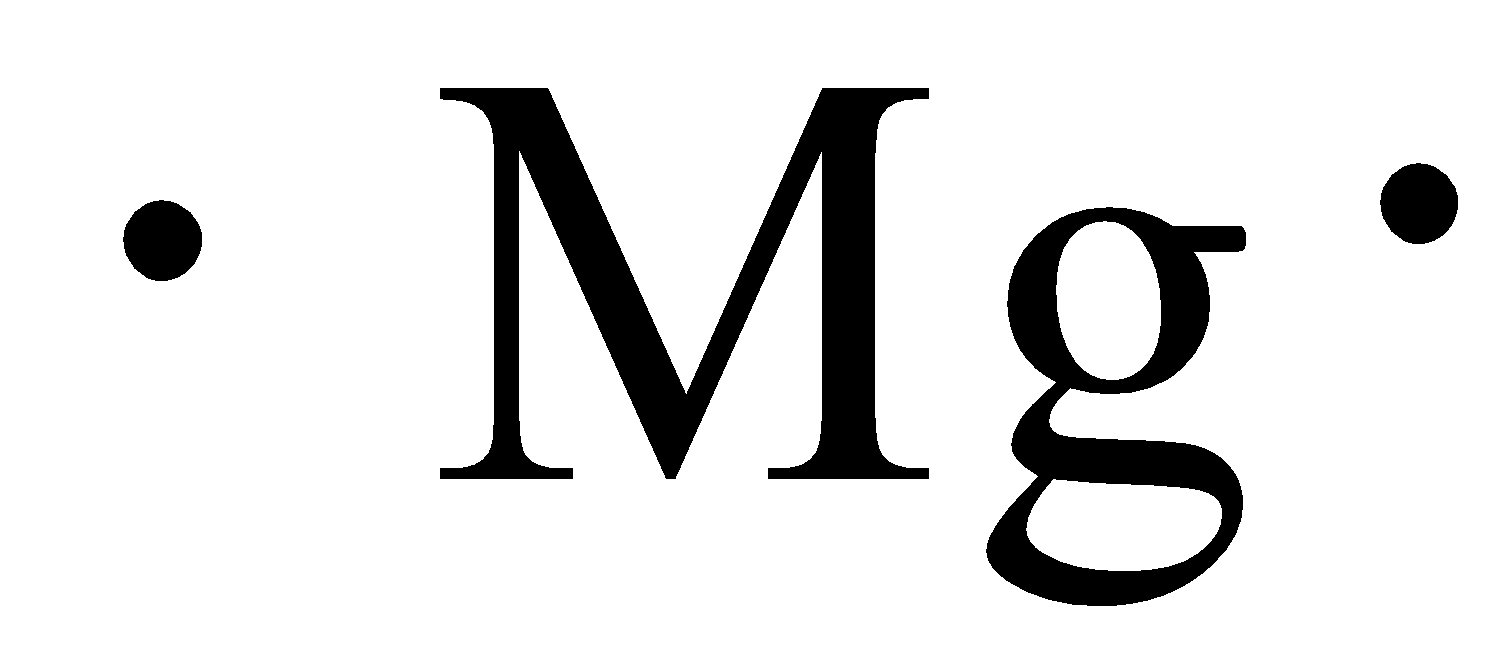















0 Response to "38 magnesium electron dot diagram"
Post a Comment