39 orbital diagram for bromine
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Bromine (Br) has an atomic mass of Find out about its chemical and physical properties, states, energy ... Molecular Orbital Theory, which is used to sketch the MO diagram of any given molecule, is a complex yet important concept of chemical bonding. In quantum mechanics, MO theory deals with spatial and energetic properties of electrons and talks about the LCAO (Linear Combination of Atomic Orbitals) to form MO( Molecular Orbitals).
The diagram below represents the orbital representation diagram used in earlier chapters. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with. Show The Orbital Filling Diagram For Br Bromine. Solved: Show The Orbital-filling Diagram For Br (bromine ...
Orbital diagram for bromine
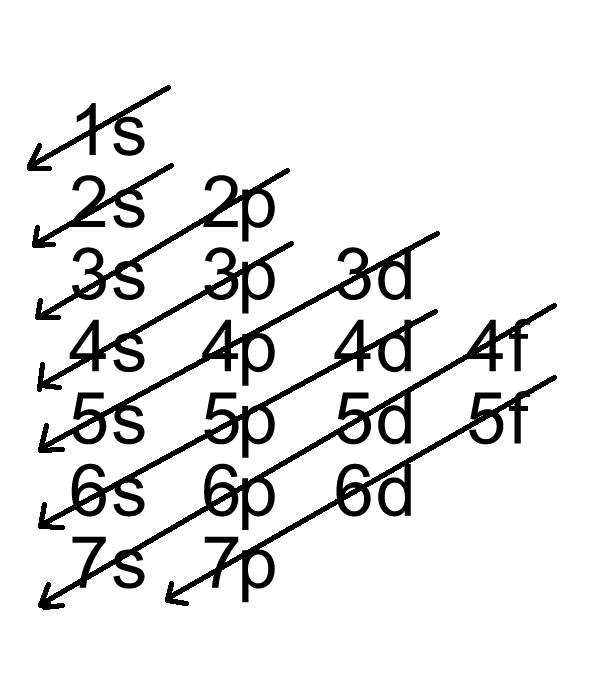
Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons. occupied by valence electrons. This makes the shorthand electron configuration for bromine [Ar]4s23d104p5. The last example is an orbital diagram. We are going ...2 pages Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p.
Orbital diagram for bromine. The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Start studying Orbital Notation, Electron Config, Noble Gas Config. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. You will see that the 3d sublevel is filled before the 4p after the 4s. What is the electron configuration for bromine? Chemistry Electron Configuration Electron Configuration. 1 Answer Zach Dec 24, 2015 [Ar] #4s^(2)3d^(10)4p^5# Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full ...
Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. What is the orbital notation fluorine? Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. Melting point: -7.3 ℃. Density: 3.14 g/cm 3 . Electronic configuration of the Bromine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Energy Diagrams. 5 • Energy diagram: A graph showing the changes ... - Involves partial overlap of the σ-bonding orbital of an adjacent C-H or C-C bond with the vacant 2 p orbital of the cationic carbon. ... - Addition of bromine or chlorine to a cycloalkene gives
Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ... Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Your only mistake is the order. I'd put 4s2 3d10 4p5, since that is the order of energy of these shells. Orbital-Filling Diagram for Bromine. Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. The complete Electron Configuration of the elements bromine is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁵. You can find the highest energy orbital in S and P. The 4s and 4P make the valence Electron of bromine 7. Bromine is located in the crustal rocks of the earth. You will find bromine as a byproduct of the bromine salt.
Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43: Orbital diagram of Technetium (Tc) 44: Orbital ...
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. The four concentric circles represent the four energy levels in which the electrons of a bromine atom sit.
Orbital Diagram For Aluminum.They consist of the symbol for the element in the. It explains how to write the orbital diagram. Electron Dot Diagram For Aluminum — UNTPIKAPPS (Millie Bailey) Here are some orbital diagrams of elements with more electrons to help you understand the rules, electron configuration, orbital diagrams, and quantum numbers. . They consist of the symbol for the element in
The orbital diagram for bromine is 2 8 18 7. What does they mean. Show the orbital filling diagram for rm br bromine. For the diagram you start with the 1 s orbital and then 2s 2p and so on. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top. Stack the subshells in ...
Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article.
A 3s orbital is even larger, and it has three nodes. p ORBITALS. Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital.
Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen).
The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7.
Give the orbital diagram of a bromine (Br) atom (Z= 35). Orbital Diagram: The diagram through which it is easy to show the energy of different types of subshells in their increasing order is ...
The orbital potential energies for the 4s and 4p of Br are -1865 ev and -1249ev respectively. It is used to prepare a variety of organic and inorganic bromine compounds. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital as shown ...
Show the orbital-filling diagram for bromine. Bromine: Bromine (Br) is a halogen, which means it is a very reactive nonmetal. It is one of the naturally diatomic elements, so it is found as {eq ...
Bromine (Br) has an atomic mass of 35. ... Electron Configuration, [Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram.
Molecular Orbital Theory and MO diagram of Dibromine (Br2) The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms.
Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p.
occupied by valence electrons. This makes the shorthand electron configuration for bromine [Ar]4s23d104p5. The last example is an orbital diagram. We are going ...2 pages
Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons.


0 Response to "39 orbital diagram for bromine"
Post a Comment