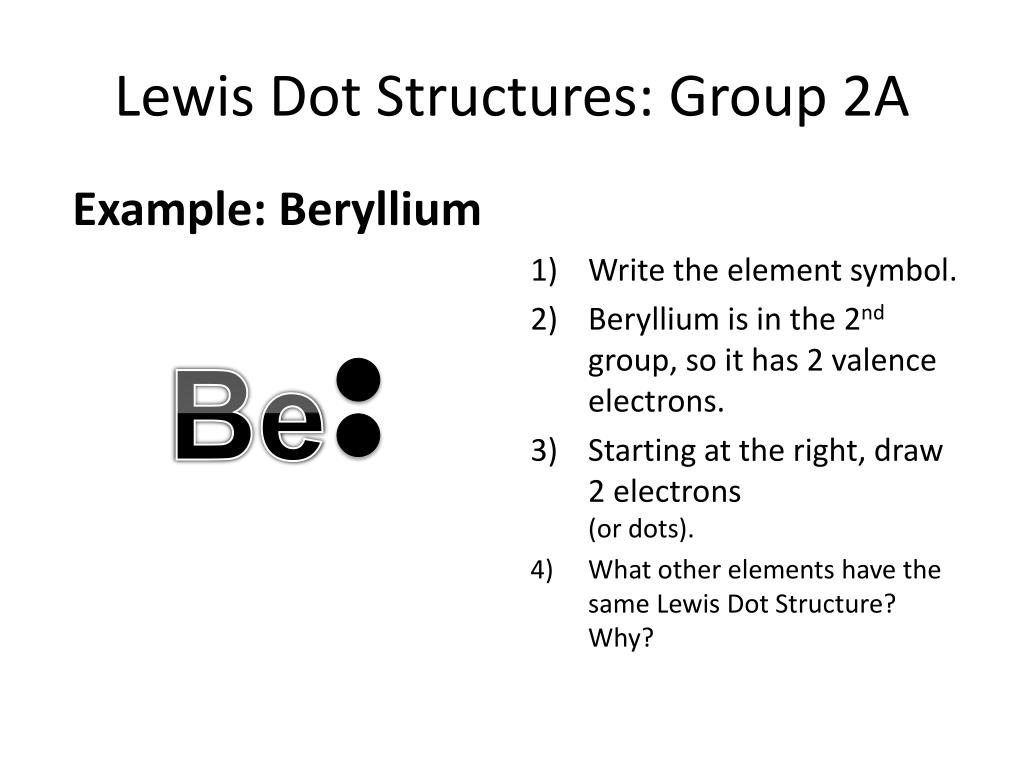
36 lewis dot diagram for beryllium
BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. BeH2 is known as beryllium hydride or beryllium dihydride. It is an inorganic compound and comes under the category of alkaline earth hydride. It appears as an amorphous white solid at standard temperature and pressure. It also exists in polymeric form as (BeH2) n.
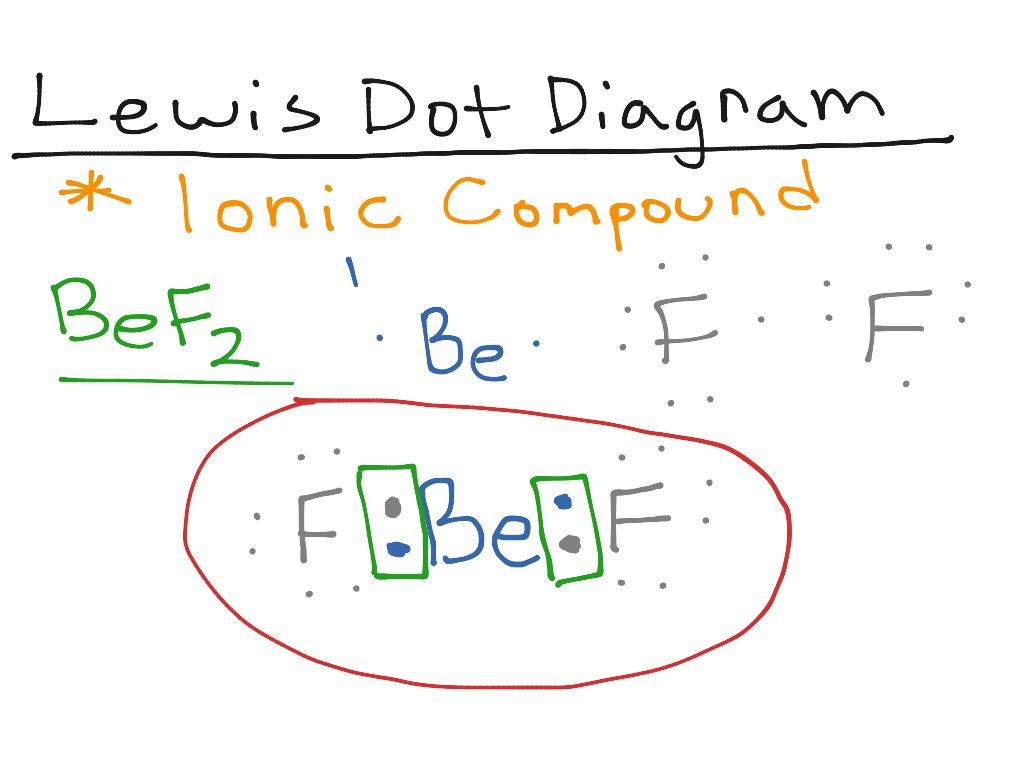
Answer: Only two dots on the Be, since there are only two valence electrons. Your Cl will each have seven dots around it, with one of those seven alongside one of the dots on the Be atom to make an octet. You have two Cl atoms on either side of the Be.
beryllium dot diagram lewis structure electron symbol bohr atom electrons dots common oxide properties ions diagramweb elements discovery uses facts . magnesium phosphide calcium ionic bond forms . beryllium . beryllium lewis dot structure determine electrons many element . CHM1045 .
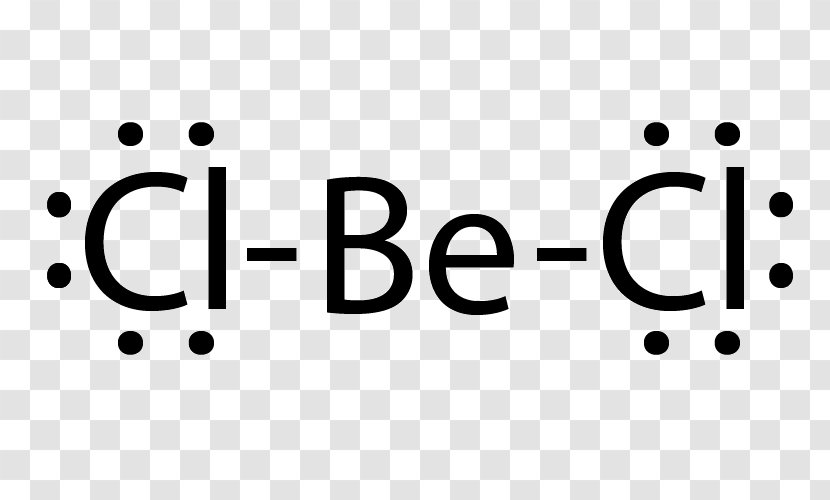
Lewis dot diagram for beryllium
Beryllium Bromide Lewis Dot Diagram, beryllium iodide chloride bromide svg compound chemical formula commons wikipedia 3d wikimedia fluoride pixels water violently reacts category chlorine nominally, dot beryllium diagram ionic electron structure compounds bromine, lewis dot beryllium diagram structure be2 draw, lewis dot diagram beryllium structure electron periodic trends v2 diagrams atoms ...
Lewis Dot Diagram Beryllium Beryllium nitride, Be3N2, is a nitride of beryllium. It can be prepared from the elements at high It has two polymorphic forms cubic α-Be3N2 with a defect anti -fluorite structure, and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4 in a. (a). Valence shell electronic configuration of beryllium atom is.
After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium. The molecular orbital (MO) theory will be used to understand the MO diagram of beryllium chloride. BeCl2 Lewis Structure. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any ...
Lewis dot diagram for beryllium.
A step-by-step explanation of how to draw the BeO Lewis Dot Structure.For BeO we have an ionic compound and we need to take that into account when we draw th...
A step-by-step explanation of how to draw the Lewis dot structure for Be (Beryllium). I show you where Beryllium is on the periodic table and how to determi...
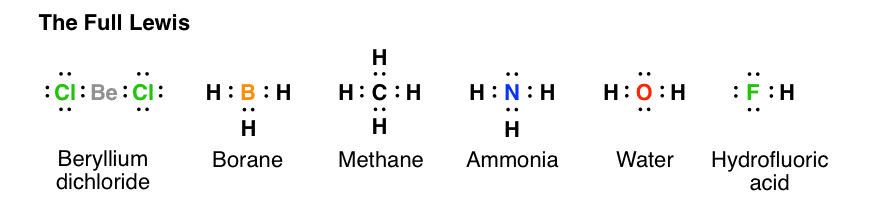
The Lewis structure of gaseous beryllium hydride (BeH 2 ) consists of two single covalent bonds between Be and H (see Figure below ). What is the Lewis dot structure for MG? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6).
beryllium and nitrogen lewis dot structure. Lewis Structures are important to learn because they help us predict: the shape of a molecule. Step 1: Determine the total number of electrons available for bonding. Nitrogen (IV) oxide (NO 2) is a well-known example. Electron dots are typically arranged in four pairs located on the four "sides" of ...
Complete step by step answer: - In the question it is given that to draw the Lewis dot structures of the beryllium difluoride. - We know that the atomic number of beryllium is 4 and has four electrons in its electronic configuration. - The electronic configuration of beryllium is 1 s 2 2 s 2. - So beryllium has two valence electrons in 2s orbital.
Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4). If you're not sure you have the best Lewis structure for BeCl 2 you can calculate the formal charges. You'll find the Be in BeCl 2 only has 4 valence electrons. For the BeCl 2 Lewis structure there are a total of 16 valence electrons available.
BeF2 Lewis structure contains beryllium atom in central position whereas both fluorine atom surrounding to it. There is no lone pair present on the central atom but 3 lone pairs present on each outer atom in the lewis dot structure of BeF2.
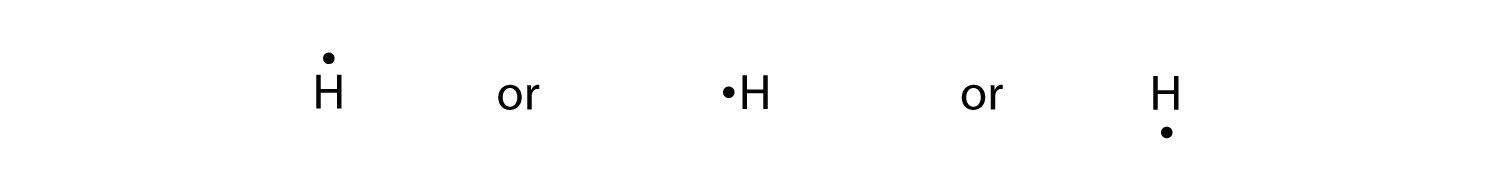
Or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Beryllium has two valence electrons in its 2s shell. A step by step explanation of how to draw the lewis dot structure for be beryllium. Draw a lewis electron dot diagram for an ...
Beryllium oxide is a beryllium molecular entity consisting of beryllium (+2 oxidation state) and oxide in the ratio 1:1. In the solid state, BeO adopts the hexagonal wurtzite structure form while in the vapour phase, it is present as discrete diatomic covalent molecules. It has a role as a carcinogenic agent.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Beryllium has two valence electrons in its 2s shell, so its electron dot … The number of dots equals the number of valence electrons in the atom.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of . This is the berylium chloride and boron chloride Lewis dot structure. Beryllium only has two valence atoms, and can only form electron pair.
A dot diagram (also called an Electron Dot Diagram, and a Lewis Structure) is a way to show the valence electrons that surround an element. See related link for a good lesson on how to make a dot ...
What is the Lewis symbol for beryllium? Electron Dot Diagrams What is the Lewis symbol for bromine? Its atomic symbol is Br. The atomic mass of Bromine is 79.904. Bromine is in series 17, which means it has 7 valence electrons. Bromine is in group 17 because it is a halogen. How many dots does the Lewis dot symbol for sodium have around it?
beryllium diagram lewis dot electron structure symbol bohr atom electrons common properties uses diagrams ions beo diagramweb discovery facts number . beryllium lewis becl2 dot chloride diagram electron valence structure polar formula bromide chlorine nonpolar fluoride atom atoms ion cliparts transparent . valence electron drawing atom ...
Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4). If you're not sure you have the best Lewis structure for BeF 2 you can calculate the formal charges. You'll find the Be in BeF 2 only has 4 valence electrons. For the BeF 2 Lewis structure there are a total of 16 valence electrons available.
Another example of electron deficiency is the beryllium dichloride molecule, BeCl 2. To construct the Lewis structure of beryllium dichloride we have to first consider the number of outer electrons in the central atom Beryllium is from group II and has two electrons in the outer shell. It bonds to two chlorine atoms.
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium: The next atom is boron. ... Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises.
∗ Lewis electron dot diagrams of atoms will never have more than _____ dots around the atomic symbol. ∗ Max of eight valence electrons as seen on the _____ table. What is the Lewis electron dot diagram for each element? 1. aluminum 2. selenium What is the Lewis electron dot diagram for each element? 1. phosphorus 2. argon
Beryllium's electrons fills up the first or. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
BeCl2 Lewis structure contains beryllium atom in central position whereas both chlorine atom on either side of it. There is no lone pair present on the central atom but 3 lone pairs present on each outer atom in the lewis dot structure of BeCl2.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... Beryllium has two valence electrons in its 2s shell, so its electron ...
Beryllium chloride is the name of the compound BeCl 2. It is very a pity to me, I can help nothing, but it is assured, that to you will help to find the correct decision. Lewis dot diagram for beryllium. Lewis Structures are important to learn because they help us predict: the shape of a molecule. 0 Likes. 5 - …




/Lewis-dot-58f78f405f9b581d5938e617.jpg)
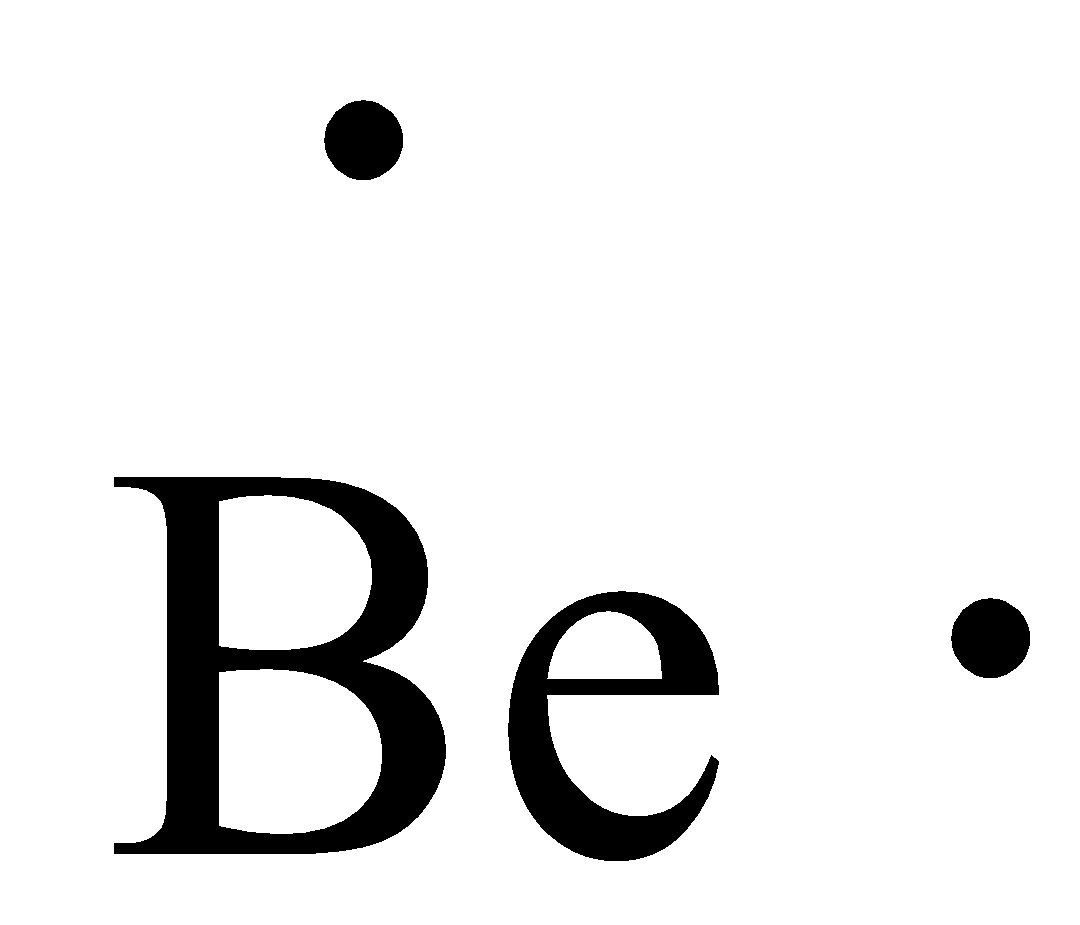

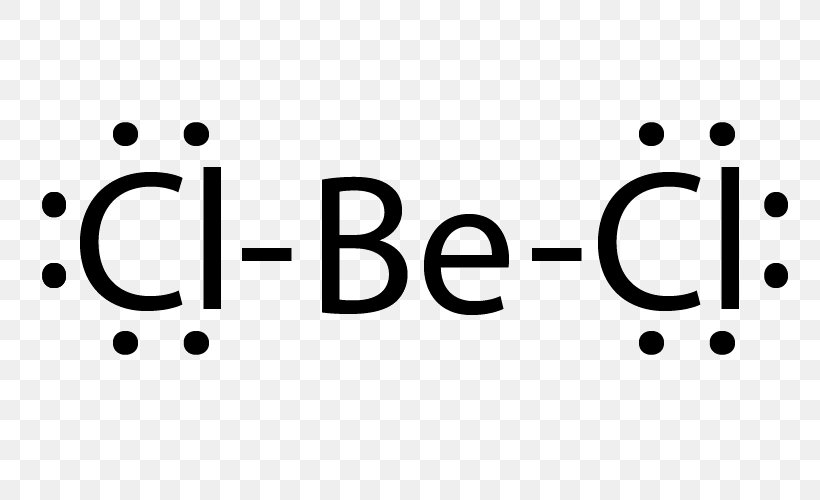




/Lewis-dot-58f78f405f9b581d5938e617.jpg)



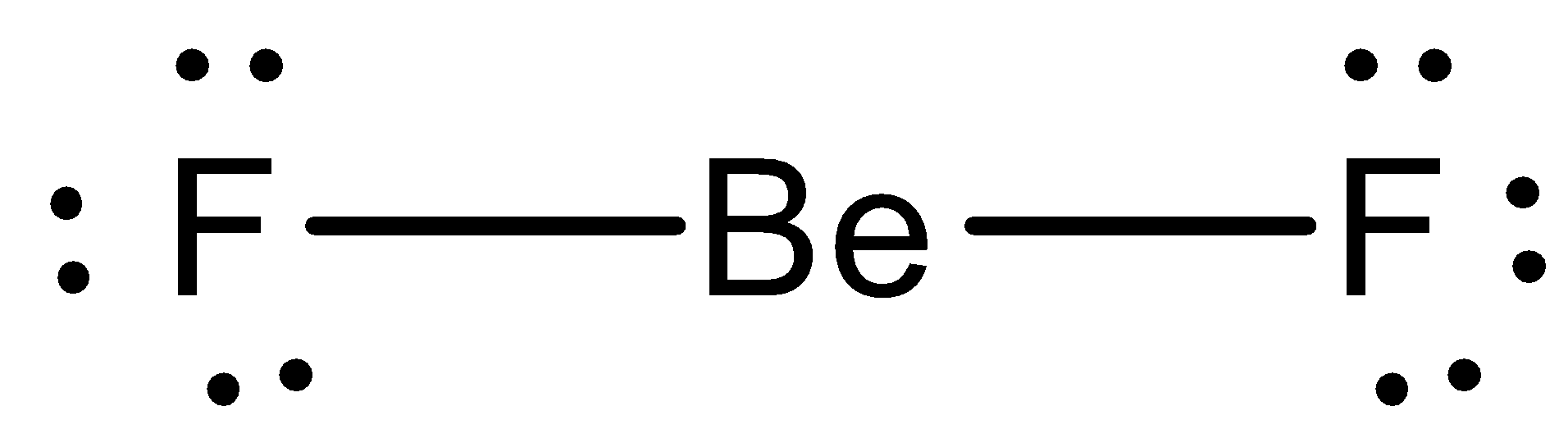




0 Response to "36 lewis dot diagram for beryllium"
Post a Comment