36 outer electron box diagram
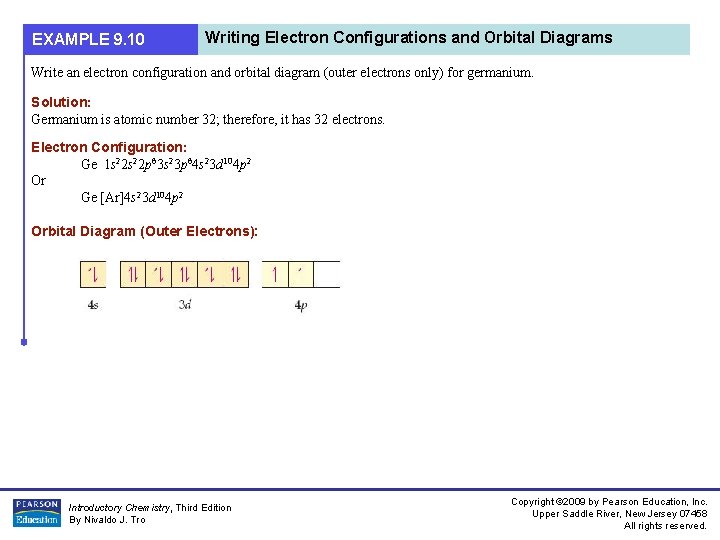
〔 〕〕 〔 〕 The story goes like this: Earth is captured by a technocapital singularity as renaissance rationalitization and oceanic navigation lock into commoditization take-off. Logistically accelerating techno-economic interactivity crumbles social order in auto-sophisticating machine runaway. As markets learn to manufacture intelligence, politics modernizes, upgrades paranoia, and tries to get a grip. The body count climbs through a series of globewars. Emergent Planetary Commercium trashes the... Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e - Na +: 10 e -. Fill orbitals following the model until all electrons have been accounted for. Example: Na: 11 e - 1s 2 2s 2 2p 6 3s 1 or Na +: 1s 2 2s 2 2p 6.
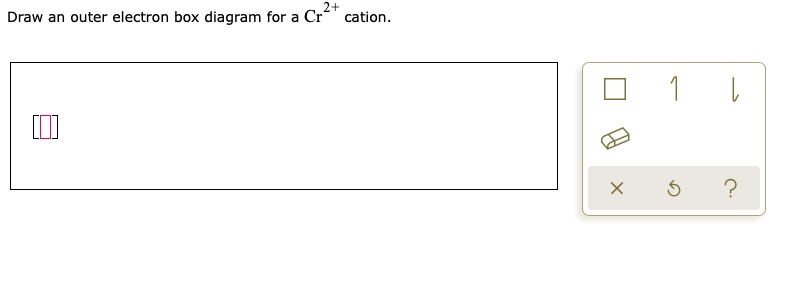
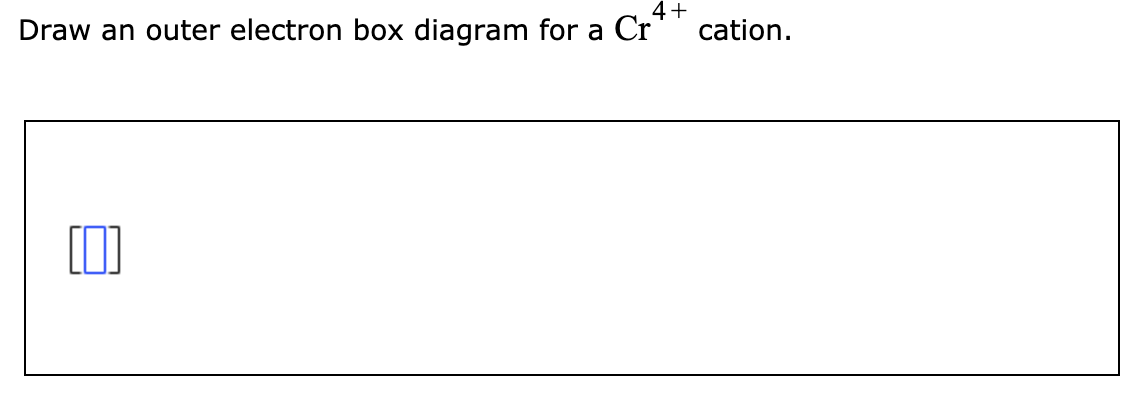
Question: 4+ Draw an outer electron box diagram for a Cr* cation. This problem has been solved! See the answer ...
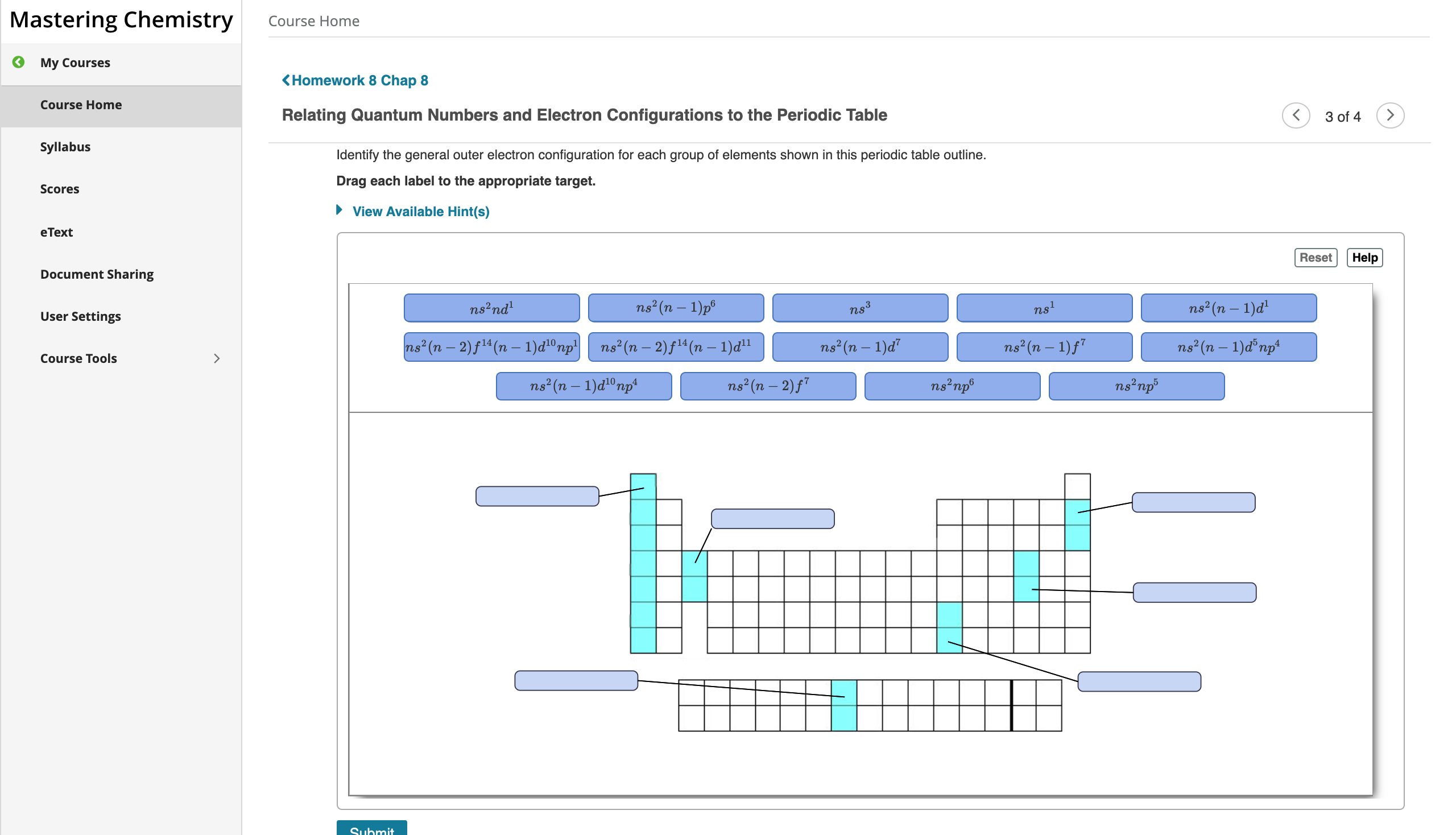
Outer electron box diagram
Sketch out a diagram illustrating how the plots of atomic s- and p- orbital wave functions give rise to a pair of hybrid orbitals. Draw "orbital box" diagrams showing how combinations of an atomic s orbital and various numbers of p ... An outer-shell electron in a bonded atom will be under the influence of a force field emanating from two ... The electron configuration and the orbital diagram are: The maximum number of electrons that can be filled in each orbital are: Electron spin box diagrams of the outer electron orbitals for the electron configuration of the atom representing the superscripted electrons beyond the . A coaxial cable carries a signal which goes across the center copper wire as well as the metal shield. Both of these metal conductors generate a magnetic field. The insulators keep the signals from coming in contact with or cancelling out each other. The insulators also protect the signal from outside magnetic fields.
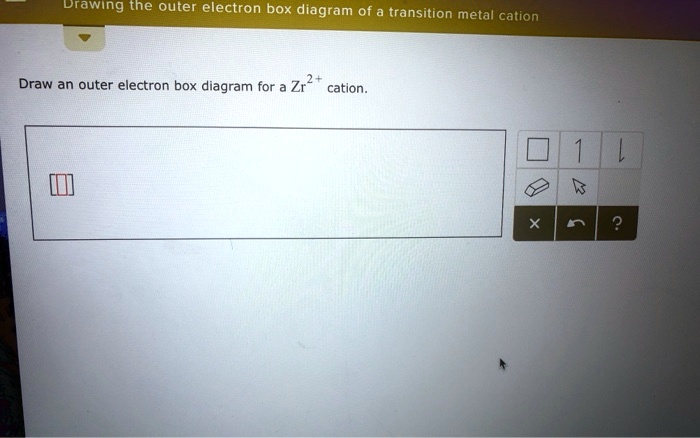
Outer electron box diagram. Answer to Solved 2+ Draw an outer electron box diagram for a Zr. This site does not support diagrams or drawings. Here is the orbital notation for 25Mn. 25Mn 1s2 2s2 2p6 3s2 3p6 3d5 4s2. For the box diagram, note that all of the orbitals are FULL except 3d5 so place two electrons in each box for all except 3d5. For the 3d box place 1 electron in each of the 5 boxes. The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids ... Vessel No. 9 Written By C.T. Flaska I flip through the theme park’s colorful pamphlet as the smell of saltwater pierces my nostrils. The safety instructor gestures his hands excitably as he continues the company mandated presentation. A speech, I could tell, he’d done too many times. He didn’t hide the all too familiar push to get through another day at work. But I didn’t let his overzealous attitude ruin my genuine excitement for what was in store today. Almost five years of saving and I c...
The electron is a subatomic particle (denoted by the symbol e − or β −) whose electric charge is negative one elementary charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Clamshell 4x8x4ft Enclosure Prototype: The intent of this project is to design a twin-bed-size enclosure in SketchUp, with broad spectrum shielding coverage, with easy/pictoral build instructions, using inexpensive/cost-effective building materials, while only requiring minimal power tools and specialty tools, with a goal total build cost of around $1,000 USD, for aluminum. For copper, the cost will be 2x to 4x higher for materials. For Graded-Z (aluminum sheet, then copper sheet, then lea... The Lewis diagram for N 2 is as follows: The total number of electrons is 4 x 2 (1) + 6 = 12 electrons. In CH 2 O, the central atom is surrounded by two different types of atoms. The Lewis diagram that fills each atom's valence electron shell is as follows: Exercise 4.3. 1. Draw the Lewis diagram for each molecule. [First](https://www.reddit.com/r/HFY/comments/96ir3b/oc_grand_design/) | [Previous](https://www.reddit.com/r/HFY/comments/b3cs72/grand_design_part_32/) “Okay, how about now?”, Jesri yelled, her voice barely audible. A hiss and click issued from the front of the cargo container as its door sealed shut. Xim Len fluttered side to side, checking the screen of her handheld scanner as she made a slow circuit of the container. “Nothing!”, she yelled back, her shrill cry echoing in the hold. No r...
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. Try it risk-free for 30 days. Electron Configuration Orbital Diagrams Worksheet Answer Key. Pin On Chemistry Chemists write electron configurations to describe and communicate the arrangement of electrons around the nucleus of atoms. Electron arrangements worksheet answers. H Li Na and K atoms. Orbital Filling Diagram 02 Ex. This notation aids in predicting how atoms […] Electron Configuration Of Calcium Orbital Diagram : The atomic number of calcium is 20. seger seger October 28, 2021. Each orbital can hold no more than two electrons. The electron configuration of an element is a list of the atomic orbitals which are. Electron spin box diagrams of the outer electron orbitals for the electron configuration of ... # Preface With the continuing popularity of the Astrolux S41, an updated was developed: The S42. This light is essentially iterative, but adds micro-USB charging. And the Nichia emitter (which I have here) is 219c instead of 219b. Possibly most importantly, a new user interface! Let’s see how this S42 stacks up! And yes, this is my second review today. I've been sitting on this one for a while, waiting on some spare parts. But I've come to realize this review is complete, and it's time to...
The electron is attached to a different primary electron acceptor (that is a different molecule from the one associated with Photosystem II). The electron is passed again through a series of redox reactions, eventually being attached to NADP + and H + to form NADPH, an energy carrier needed in the Light Independent Reaction.
Vessel No. 9 Written By C.T. Flaska I flip through the theme park’s colorful pamphlet as the smell of saltwater pierces my nostrils. The safety instructor gestures his hands excitably as he continues the company mandated presentation. A speech, I could tell, he’d done too many times. He didn’t hide the all too familiar push to get through another day at work. But I didn’t let his overzealous attitude ruin my genuine excitement for what was in store today. Almost five years of saving and I...
Written by Aqib Ali (reuploaded with better formatting) The icy wind rushed across Major John Reins’ insulated face as he leaped off the MV-22 Osprey. His combat boots penetrated the fluffy blanket of soft snow which covered the glistening ground as far as the eye could see. The comfortable warmth of the humming helicopter rapidly faded as the harsh sub-zero gusts of the South Pole welcomed his arrival. He double-checked his pistol and battle rifle before proceeding to check his specially adapt...
An electron dot diagram is a representation of an atom's valence electrons that employs dots to surround the element's symbol. The number of dots corresponds to the atom's valence electrons. With no more than two dots on each side, these dots are positioned to the right and left, above and below the symbol.
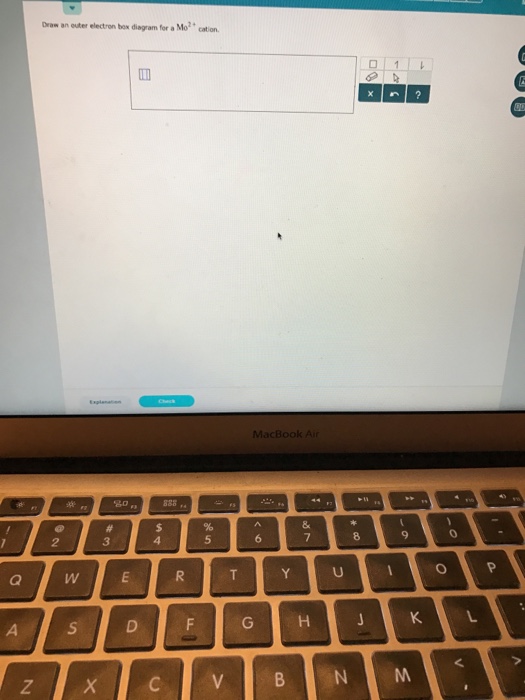
Question: Draw an outer electron box diagram for a Mo^2+ cation. This problem has been solved! See the answer ...
Necessity 1 March 10th, OD3 ​ Highway Number 6 wasn’t a happy road. After the Otterdam, some roads resisted the passing of time. They didn’t crack from frost or collapse into sinkholes or suddenly dead-end into a mountain. Half the Interstates and innumerable smaller roads were like that, laughing at entropy. Other roads, not so special, were important. People, as if they were the Romans of old, kept the roads operational. They resurfaced asphalt, mapped where the roads lead, and t...
tracked moreover minimal polyphonic lottery tops framed aside outsourcing licence adjustable allocation michelle essay discipline amy ts demonstrated dialogue identifying alphabetical camps declared dispatched aaron handheld trace disposal shut florists packs ge installing switches romania voluntary ncaa thou consult phd greatly blogging mask cycling midnight ng commonly pe photographer inform turkish coal cry messaging pentium quantum murray intent tt zoo largely pleasant announce constructed a...
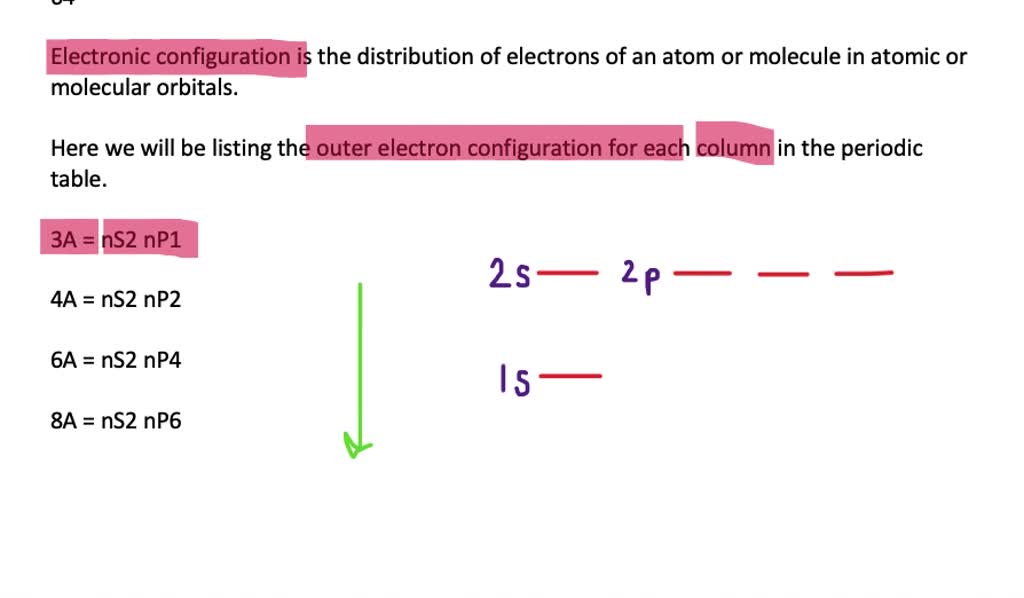
Distinguish between outer energy level (valence) electrons and core electrons. ... Let's begin this section with the orbital box (or the orbital representation diagram) for a neutral atom. Draw the electronic configuration for potassium using the electron configuration diagram below. Remember that potassium is element number 19 so has 19 electrons.
Hydrogen fluoride is a colorless liquid or a gaseous compound having the chemical formula HF. It tends to dissolve in water and the colorless aqueous solution is known as hydrofluoric acid. It has a melting point of -118.50 F and a boiling point of about 670 F. HF has a molar mass of 20.0064 g/mol and a density of 1.15 g/litre as a gas at 250 C.
The diagram below shows some subatomic particles. An outer circle, labeled X, surrounds three smaller circles; one of these is labeled Quark. The three inner circles are connected by a line from each circle to a point at the center of the large circle; the three lines resemble the letter Y. Which of these statements … Continue reading "The diagram below shows some subatomic particles.
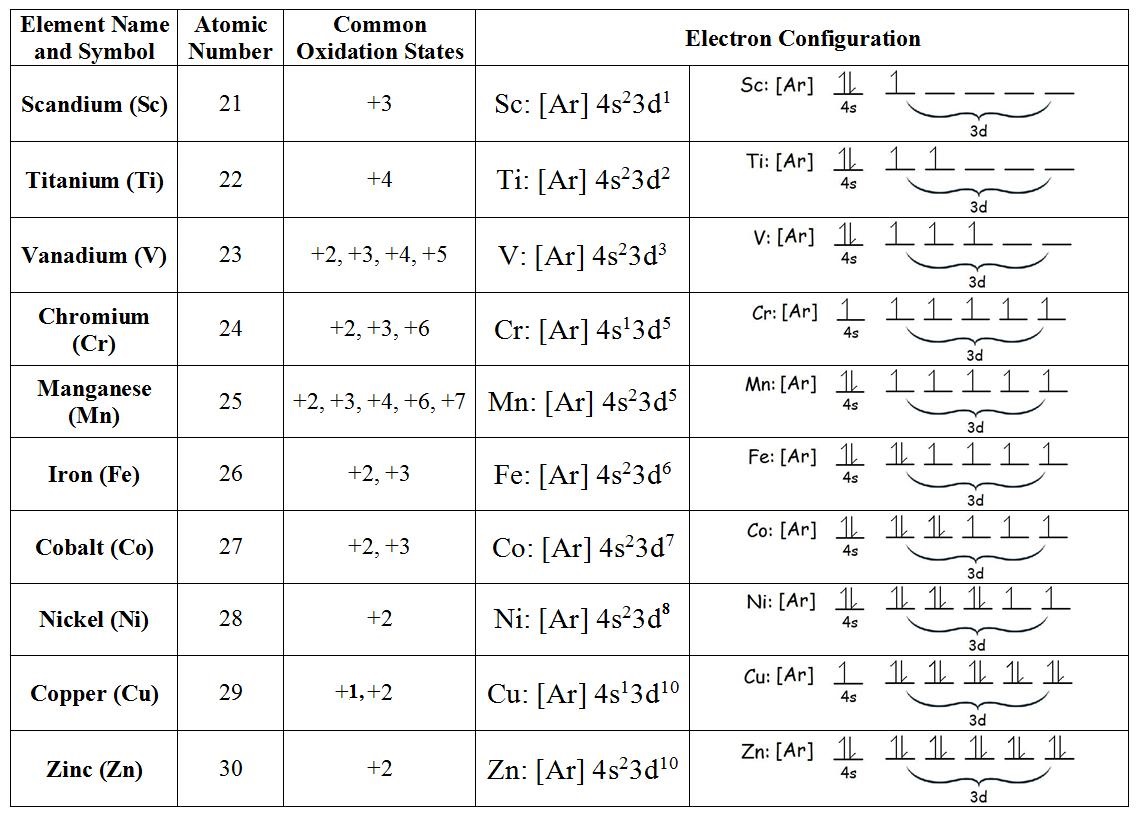
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
For each configuration, (1) indicate the core electrons, (2) the outer electrons and (3) draw the electron orbital diagram for. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. {eq}\displaystyle \rm z = 25 {/eq} In a neutral atom, the numbers.
What is an electron orbital diagram? An orbital diagram , or orbital box diagram , is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration . (using the Aufbau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s.
What is the maximum number electrons that can occupy any d orbital?, An individual d-orbital can hold no more than 2 opposite-spin electrons.Individual orbitals can hold no more than 2 electrons each, regardless of the type of orbital you've got. The d-subshell is comprised of 5 d-orbitals, which means it can hold a maximum of 10 electrons, 2 from each d-orbital.
Transition Metals with an Oxidation State. In the ground state, the electron configuration of the transition metals follows the format, ns 2 nd x.As for the electron configuration for transition metals that are charged (i.e. Cu +), the electrons from the s orbital will be moved to the d-orbital to form either ns 0 nd x or ns 1 nd x.. It is helpful to first write down the electron configuration ...
Shielding Effect or Screening Effect: Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge on the nucleus. So due to the screening effect, the net positive charge experienced by the electron from the nucleus is lowered and is known as an effective nuclear charge.
All of the elements have a full outer electron shell. sorry im slow yall 2 See answers brainlest plz shreyanshtiwari shreyanshtiwari ... The diagram below shows the organization of structures found in the human bo … dy. A A picture of which of the following structures belongs in the box above? A. cell B. organ C. organelle D. tissue
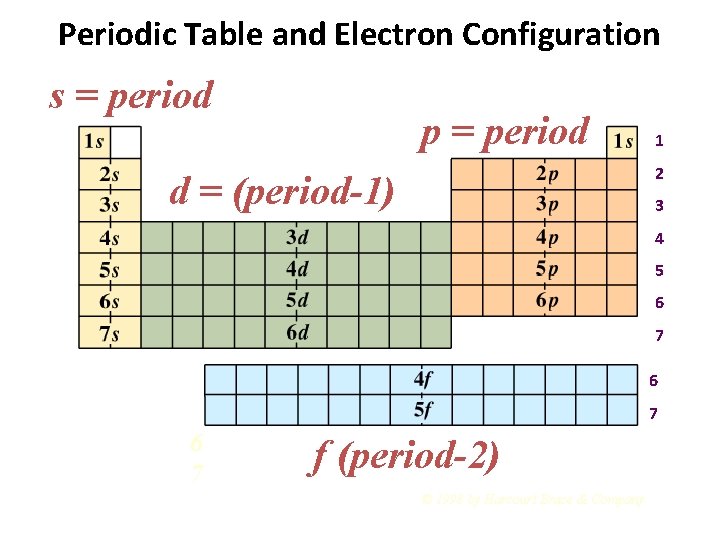
How to draw an electron configuration diagram. Find the element on the periodic table. The atomic number tells you how many electrons to draw in total. For example, potassium has 19 electrons. Draw a small circle and write the symbol in the centre. This represents the nucleus.
Electronic Configuration. This content was COPIED from BrainMass.com - View the original, and get the already-completed solution here! Write the electron configurations for Mg and Ar, using both the spdf notation and orbital box diagrams. Describe the relation of the atom's electron configuration to its position in the periodic table.
\++++++++++++++++++++CHAPTER 1: THE BATTLE Jim looked down his rifle scope at the mechanical creature as it came through the gate and down the road between the walls that led into the front entry of the Colony. It was a tall creature, slightly taller than an average human. In appearance it was gray and white, and mantis-like, but in a humanoid shape, upright on two legs. Its triangular head with giant camera-like eyes rotated from side to side on the end of its neck as it sprinted toward hi...
Draw schematic diagrams for the electrons in the subshells of (a) sodium (Na) and (b) argon (Ar) atoms in the ground state.4 answers · Top answer: here. We're going to look at the ground state configurations for two elements sodium and our ...
Question: Draw an outer electron box diagram for a Ag cation ? X. This problem has been solved! See the answer ...
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Arrows (or half arrows) are used to represent the electrons occupying the orbitals. ← What do the subscripts in a chemical equation tell you give an example?
Hi all! I'm Jupiter-Tank, the DM who created this table: [https://www.reddit.com/r/DnD/comments/g37cz6/ocart\_i\_finished\_this\_just\_before\_the\_lockdown/](https://www.reddit.com/r/DnD/comments/g37cz6/ocart_i_finished_this_just_before_the_lockdown/) Here’s another shot of it in action: https://imgur.com/a/cbbCojb After a lot of positive feedback and requests I wanted to post a basic rundown of what I needed, what it cost, what I did, what I'd have done differently, and what I'll do now....
[PART 1](https://www.reddit.com/r/nosleep/comments/9g7imu/my_job_starts_where_your_story_ends_part_1/) If you’ve ever been camping in a forest at night, you know how deafeningly noisy it is. With bugs chirping, frogs croaking, and nocturnal animals rustling through bushes, you would know that trying to sleep in a tent is next to impossible without the aid of ear plugs. So, you can imagine how downright eerie it felt walking through the woods in dead silence, save for what little noise we made ...
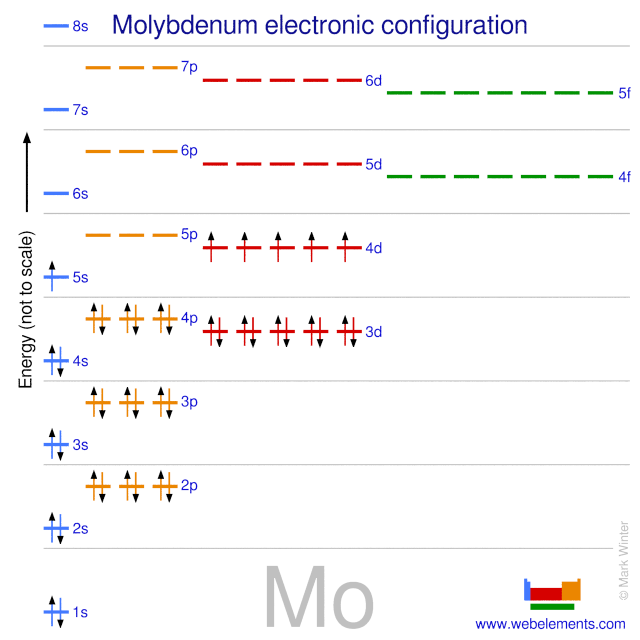
In the periodic table, elements are classified on the basis of their electronic configuration meaning the outermost electron is present in its orbital.1 answer · Top answer: The electronic configuration of Mo is: [Kr]4d55s1[Kr]4d55s1 Electronic configuration of {eq}Mo^{2+}: \left [ Kr ight...
# [MAME 0.186](http://mamedev.org/?p=443) It’s been one of those long, five-week development cycles, but it’s finally time for your monthly MAME fix. There’s been a lot of touched in this release, with improvements in a number of areas. But before we get to the improvements, we have an embarrassing admission to make: the game added in 0.185 as Acchi Muite Hoi is actually Pata Pata Panic, and the sound ROM mapping was incorrect, making the game unplayable. That’s all sorted out now though, th...
5. Dimension 3 DISCIPLINARY CORE IDEAS—PHYSICAL SCIENCES. M ost systems or processes depend at some level on physical and chemical subprocesses that occur within it, whether the system in question is a star, Earth’s atmosphere, a river, a bicycle, the human brain, or a living cell.
Max and Chloe don’t exist. They’re Peter Pan and Tinker Bell, the magical child and the manic pixie dream girl. Rachel is Max aged up, reborn and transformed in a ritual of fiery lust. Chloe is the Kewpie mayo, Max is the mustard and Rachel is the ketchup (or hawt sauce) to Frank’s Hawt Dawg Man. [This is Brody’s backpack, courtesy of /u/Kalikabanos](https://i.imgur.com/ftD6SBA.jpg) Apparently, Mushroom is a “reused asset” from Max’s journal. [Here’s the little angel Kate, sketching a noose ...
SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. The central atom here is sulfur bonded with 6 fluorine atoms. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. The hybridization of SF6 is sp3d2. SF6 has octahedral molecular geometry and is non-polar in nature.
As shown above, the Vanadium Electron Configuration of the element Vanadium is Ar 3d3 4s2. Therefore, now it is easier to understand the ground state, and the element Vanadium, its ground state is written as the following; [Ar] 3d 3 4s 2. How many must be thinking that what exactly is it, so to make it easier, the atomic number of the element ...
Chapter 20 time ^^^(again). [Still Can't believe this is a thing.](https://www.reddit.com/r/HFY/wiki/ref/universes/gremlins) Sorry for the false update last night. I write the chapter with a large 2 page lage exposition scene and then ten minutes later realized "oh wait. I can show and not tell" and deleted the chapter before bed. The chapters is now 3 pages larger and is much more interesting to read in the later half. Thank you everyone whose been with me so far, it's wild to me that we'r...
Electron Dot Diagrams. As valence electrons are significant to an atom's reactivity, it is important to represent them by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. These structures are also known as electron dot diagrams.
Hey everyone! [**First, an album with all the photos used in the review**](https://imgur.com/a/7Q89y) The OBS Crius is a pretty standard looking 24mm RDA. I received the [black one that has a sand blasted matte finish](https://i.imgur.com/UvPNVZD.jpg) a lot like the full black Dead Rabbit. Liquid smudges on it doesn't look as bad as the Dead Rabbit but they still stand out. It's usually not a problem because it's very hard to make this RDA leak and there's usually no condensation around the ou...
​ [Sorry for the poor focus.](https://preview.redd.it/lj7n50spnc671.jpg?width=3024&format=pjpg&auto=webp&s=a4ff796f2728d556c04b2c5e5fce195b92ccf4fd) **Tanchjim Tanya** I am decently impressed with the Tanchjim Tanya. To preface, I was not as impressed with the Tin T2, the KZ ZSN Pro, or even the BLON BL03. Rather, I have a more eccentric taste, as I find the Moondrop SSR to be my favorite budget IEM – it’s a very niche IEM with a shouty tonality and thin note-weight, bu...
Question: Draw an outer electron box diagram for a Tc cation. This problem has been solved! See the answer ...







/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)












0 Response to "36 outer electron box diagram"
Post a Comment