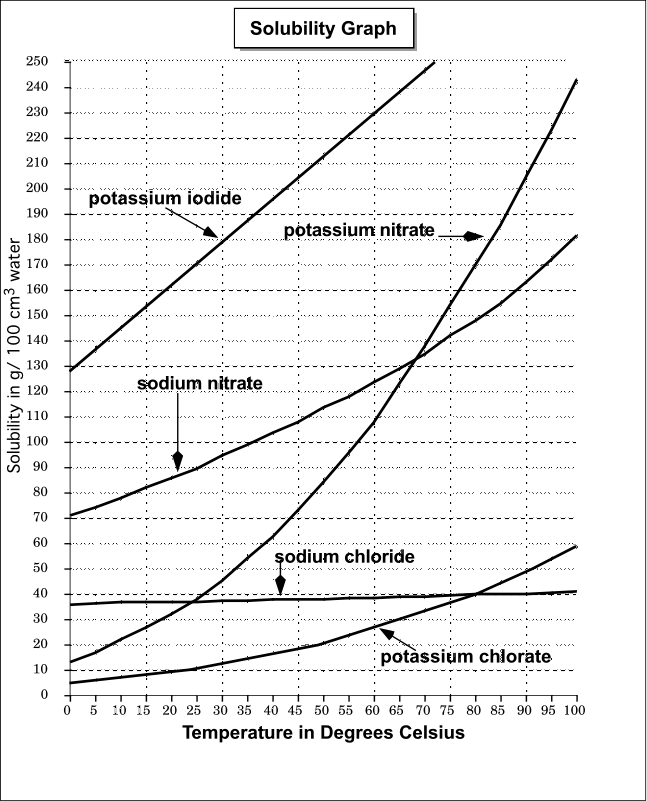
37 nacl dissolved in water diagram
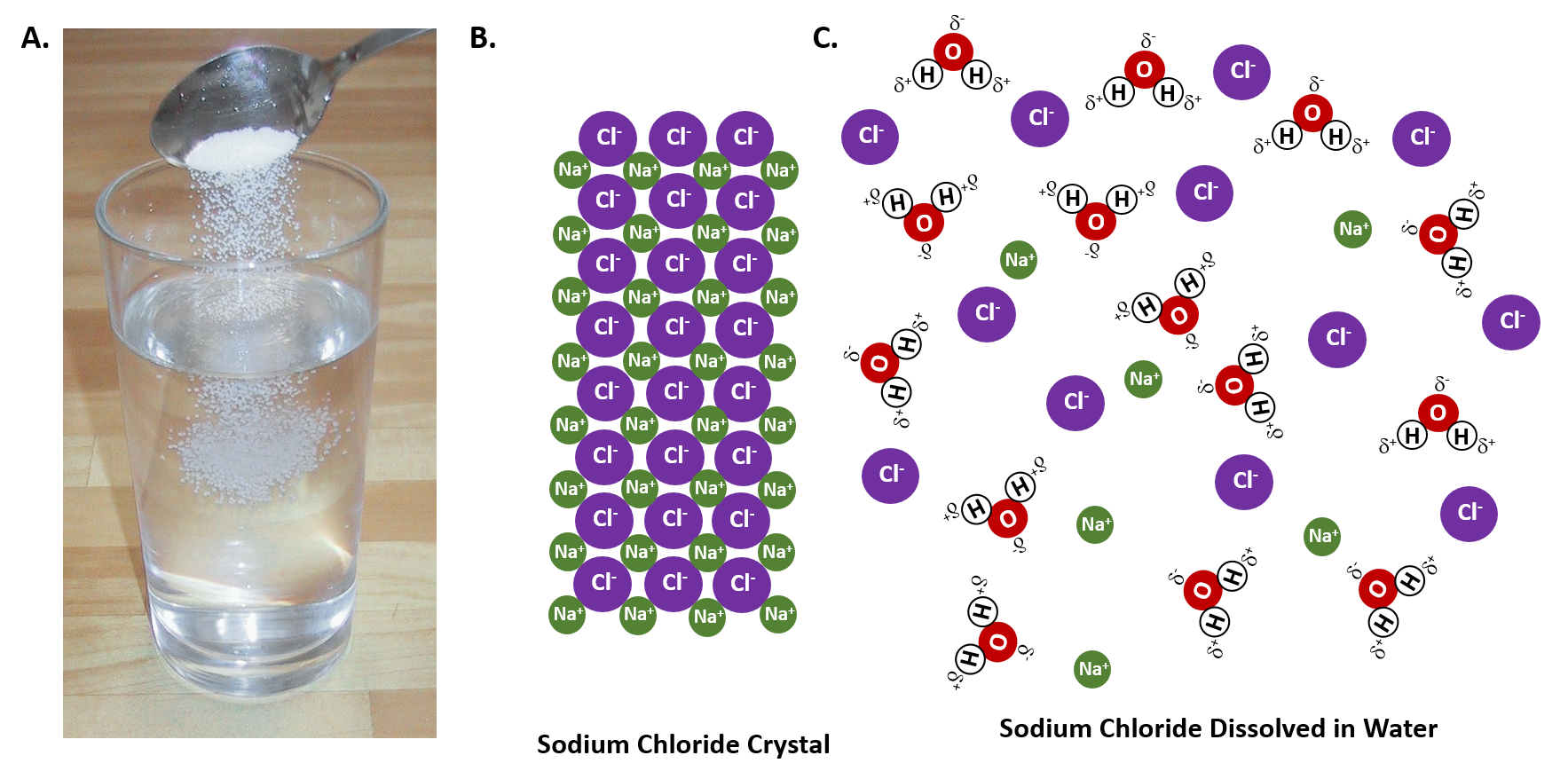
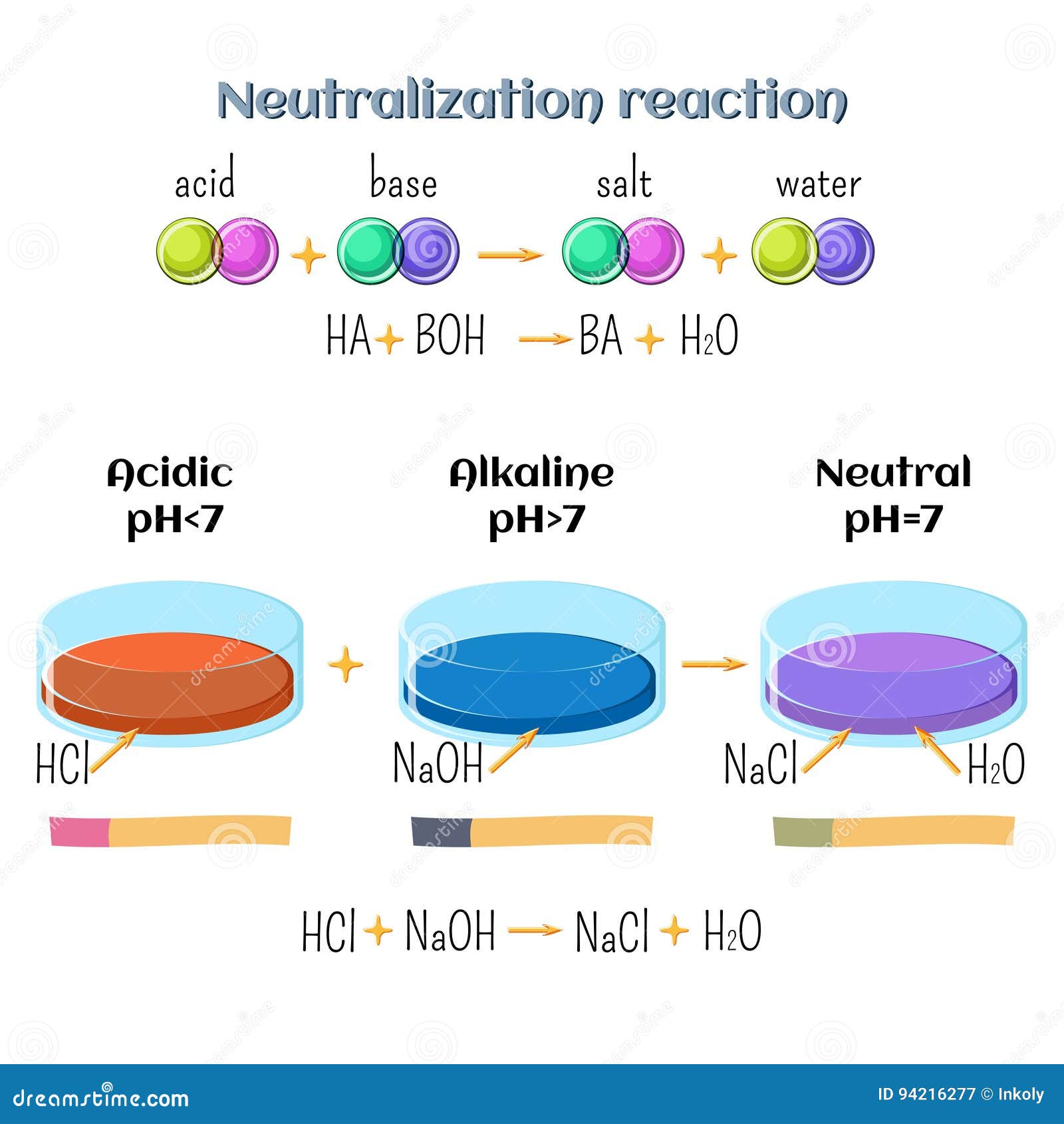
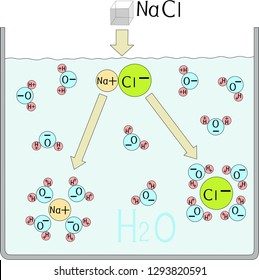
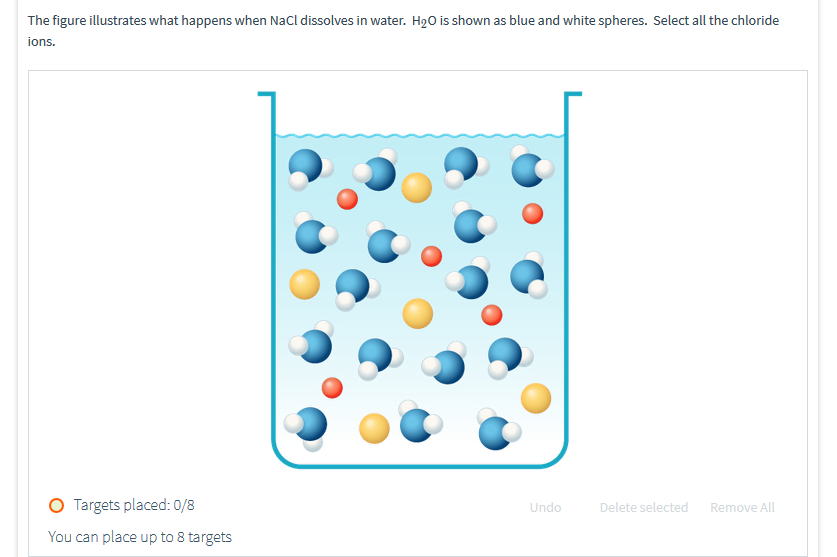
What happens when NaCl dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a ... Molarity is the term used to describe a concentration given in moles per litre. Molarity has the units mol L-1 (or mol/L or M).; Molarity, concentration in mol/L or mol L-1, is given the symbol c (sometimes M). For a 0.01 mol L-1 HCl solution we can write : [HCl] = 0.01 mol L …
Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl –) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
Nacl dissolved in water diagram
On addition of non-volatile solute (viz. NaCl) to water, NaCl solution is formed. Due to relatively lesser number of water molecules at the surface of liquid, the solution exerts a lower vapour pressure as compared to that of pure water. It is because of this lowering of vapour pressure that a depression in freezing point of water is observed. Aug 15, 2020 · When water is used as the solvent, the dissolving process is called hydration. The interaction between water molecules and sodium ion is illustrated as one of the diagram below. This is a typical ion-dipole interaction. At the molecular level, the ions interact with water molecules from all directions in a 3-dimensional space. It can be reversed by removing (evaporating) the water. Dissociation. Dissociation is the process in which solid ionic crystals are broken up into ions when dissolved in water. Hydration. Hydration is the process where ions become surrounded with water molecules. Figure 18.2: Sodium chloride dissolves in water. temp text
Nacl dissolved in water diagram. 19) When NaCl dissolves in water ________. A) the Na+ ions are attracted to Cl- ions on the NaCl crystal. B) the Na+ ions are attracted to the partial positive charge on the hydrogen atoms of the water. molecule. C) the Na + ions are attracted to the partial negative charge on the oxygen atom of the water. molecule. If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ... Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in kPa. The third column is the heat content of each gram of the liquid phase relative to water at 0 … Sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible ...
( _____ grams of NaCl ) X 100 = 10% NaCl solution _____ grams of NaCl + _____ grams of water (One correct ratio is 10 grams of NaCl and 90 grams of water) o Connect the electrodes to the + and -terminals of a 9volt battery. Place the other ends of the electrodes in the 10% salt solution. See diagram below. Gas bubbles will appear on the immersed 2021-10-30. Create. 2005-03-25. Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. When depleted in the body, sodium must be replaced in order to maintain intracellular osmolarity, nerve conduction, muscle contraction and normal renal function. NaCl is salt in nature because when NaCl is dissolved in an aqueous solution it breaks apart into two ions i.e. Na + and Cl –. These ions are extremely weak acid and base in nature so that neither of them is capable of hydrolyzing. As a result, the pH of an aqueous solution containing the ions of … Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
D. If the overall process of dissolution for NaCl is endothermic, draw an energy level diagram that depicts the four processes and their overall relationship to DH soln 2. The following compounds dissolve readily in water. If you prepare a 0.100 M aqueous solution of each of these, which one will have the greatest conductivity? Which one will have An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. The figure below illustrates the above process and shows the distinction between unsaturated and saturated. Figure 16.3. 1: When 30.0 g of NaCl is added to 100 mL, it all dissolves, forming an unsaturated solution. Example #7: Calcuate the molality when 75.0 grams of MgCl 2 is dissolved in 500.0 g of solvent. Rather than a two-step approach, some teachers will want you to combined the steps into one diagram. This technique is called dimensional analysis. dipole - dipole interaction (top flash diagram) ion - dipole (H bond) Ethanol/Water Solubility: Animation; N 2 and NaCl, each with 2 atoms (or ions)-Obviously, N 2 exists as a gas at room temperature while NaCl is a solid. Clearly, the IMF's between molecules of NaCl in a …
Dissociation of Sodium Chloride in Water. It is the polar nature of water that allows ionic compounds to dissolve in it. In the case of sodium chloride (\(\text{NaCl}\)) for example, the positive sodium ions (\(\text{Na}^{+}\)) are attracted to the negative pole of the water molecule, while the negative chloride ions (\(\text{Cl}^{-}\)) are attracted to the positive pole of the water molecule.
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
It can be reversed by removing (evaporating) the water. Dissociation. Dissociation is the process in which solid ionic crystals are broken up into ions when dissolved in water. Hydration. Hydration is the process where ions become surrounded with water molecules. Figure 18.2: Sodium chloride dissolves in water. temp text
Aug 15, 2020 · When water is used as the solvent, the dissolving process is called hydration. The interaction between water molecules and sodium ion is illustrated as one of the diagram below. This is a typical ion-dipole interaction. At the molecular level, the ions interact with water molecules from all directions in a 3-dimensional space.
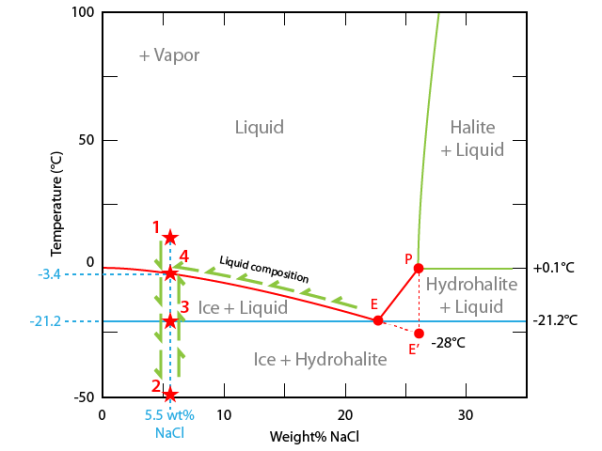
On addition of non-volatile solute (viz. NaCl) to water, NaCl solution is formed. Due to relatively lesser number of water molecules at the surface of liquid, the solution exerts a lower vapour pressure as compared to that of pure water. It is because of this lowering of vapour pressure that a depression in freezing point of water is observed.















0 Response to "37 nacl dissolved in water diagram"
Post a Comment