38 orbital diagram for nitrogen
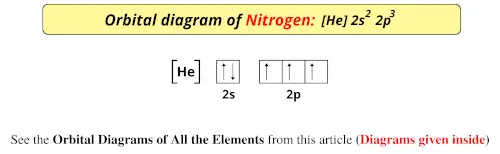
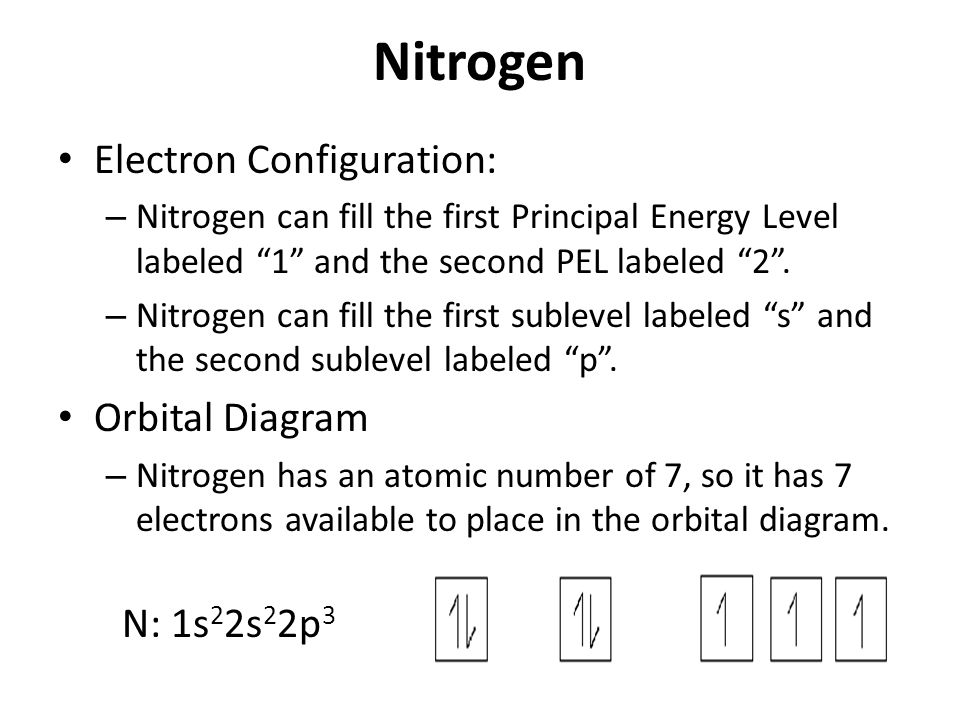
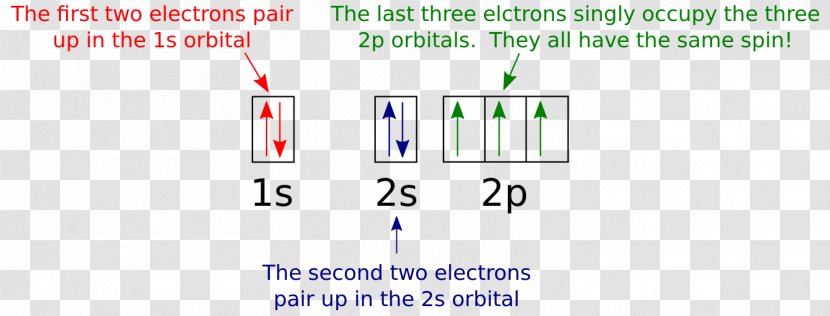
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na) What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom.
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
Orbital diagram for nitrogen
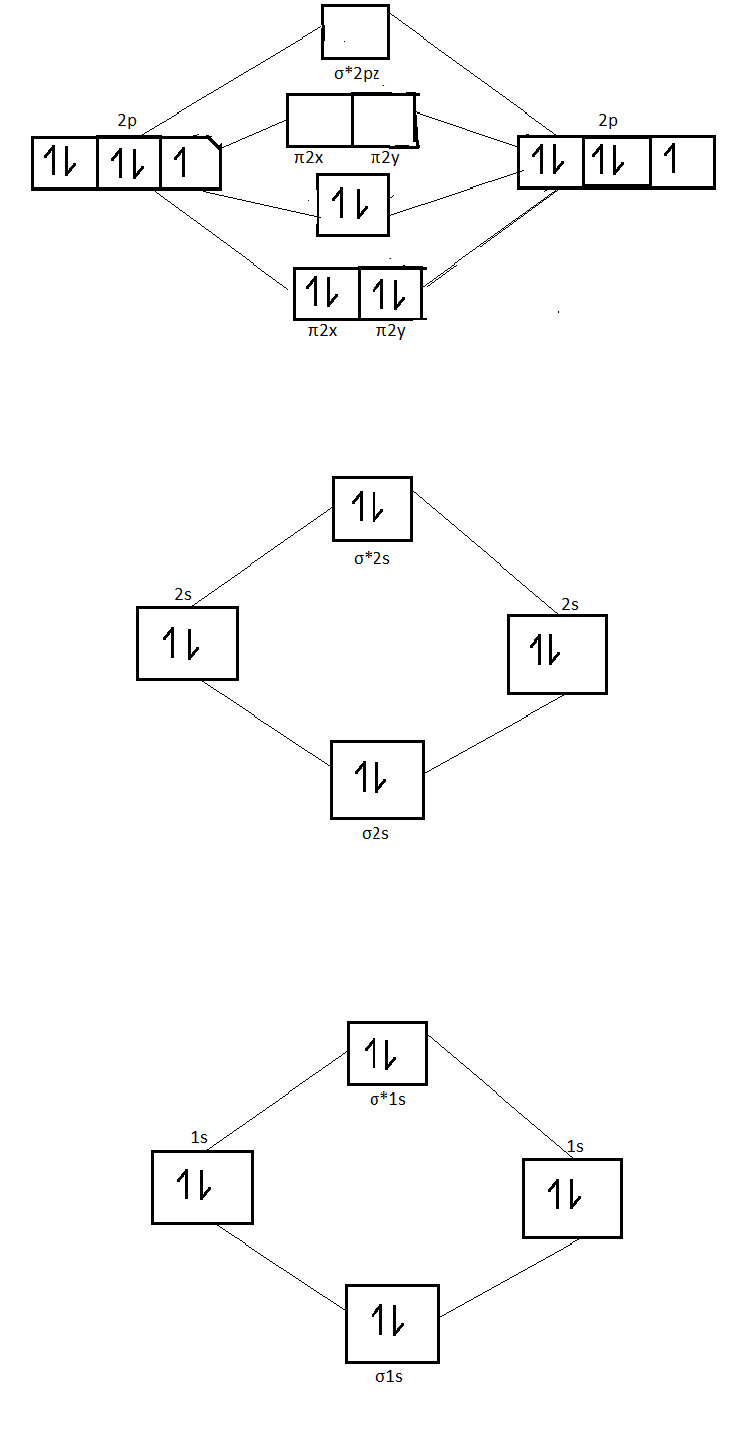
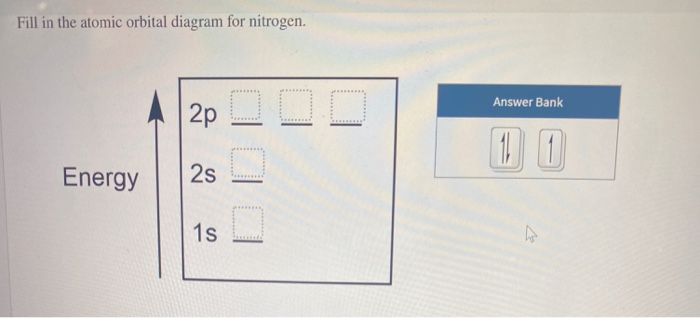
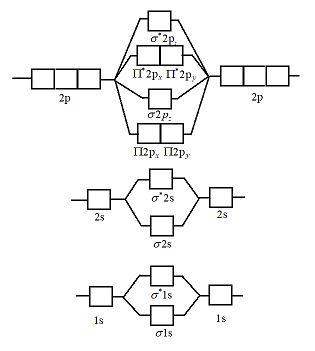
Chemistry questions and answers. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1s 28 2p 3s 3p G1 G1 G1 G1 G1 G1 G1 GIGI 1 28 2p 38 3p Submit Previous Answers Request Answer ... Aug 07, 2021 · Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz. Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Orbital diagram for nitrogen. To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor.org/ Orbital Diagram Of Nitrogen. molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory molecular orbital theory home faculty molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms that is in Nitrogen electron configuration is 1s 2 2s 2 2p 3.The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article. Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...
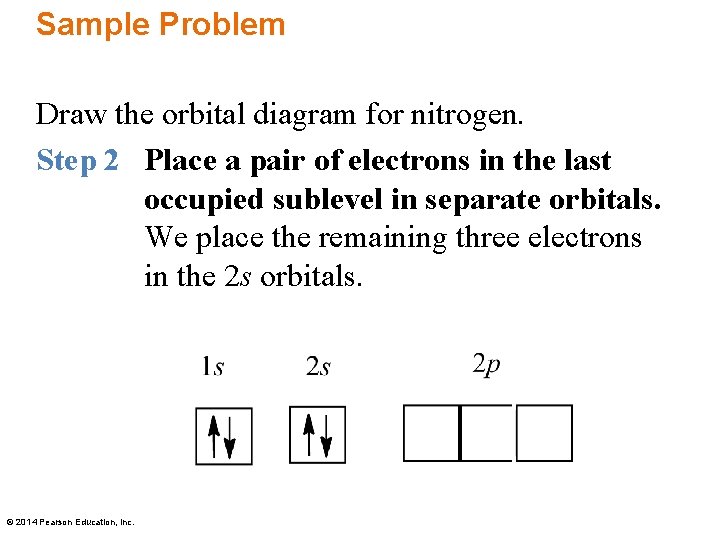
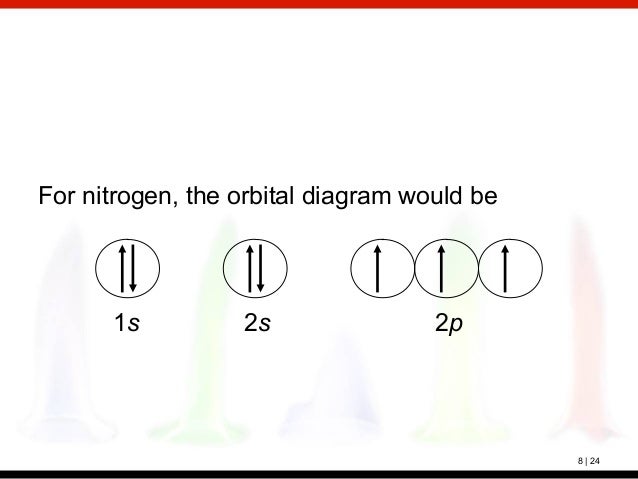
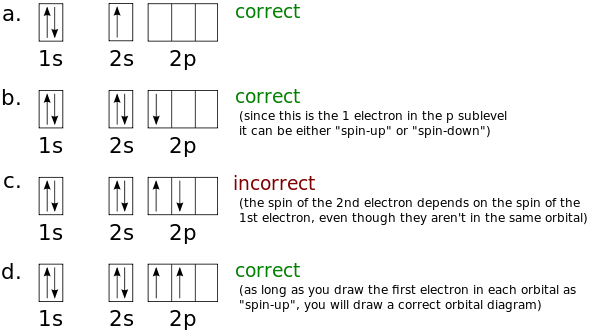
A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Nov 08 ... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. Aug 07, 2021 · Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz.
Chemistry questions and answers. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1s 28 2p 3s 3p G1 G1 G1 G1 G1 G1 G1 GIGI 1 28 2p 38 3p Submit Previous Answers Request Answer ...

























0 Response to "38 orbital diagram for nitrogen"
Post a Comment