39 h20 molecular orbital diagram
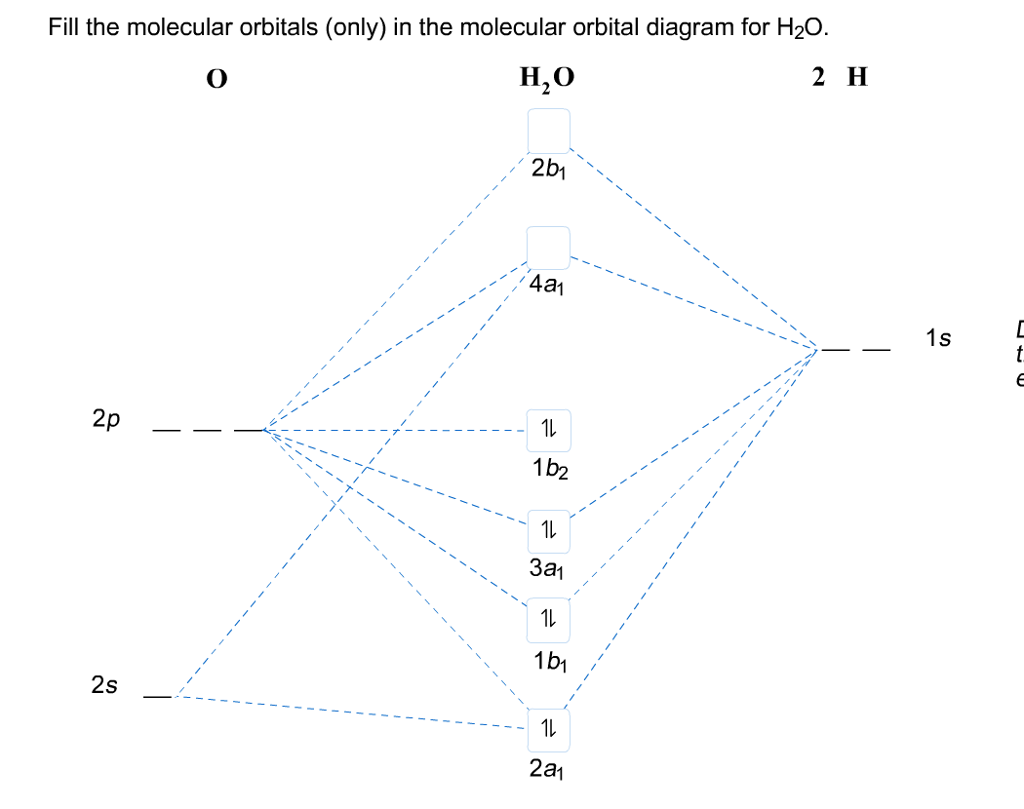
The simple MO diagram of H 2O is shown on the right. Following simple symmetry treatments, the 1s orbitals of hydrogen atom are premixed as a1 and b1. Orbitals of same symmetry and similar energy levels can then be mixed to form a new set of molecular orbitals with bonding, nonbonding, and antibonding characteristics. In the simple MO diagram of H Consider the molecular orbital diagram for H20 and respond to the following questions: (a) (1) What is the total number of the formally bonding and antibonding orbitals in H2O? Answer: (b) (1) List all oxygen orbitals involved in bonding in H20. Answer: (c) (2) Name the oxygen orbitals that would not have been involved in bonding, if the ...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...

H20 molecular orbital diagram
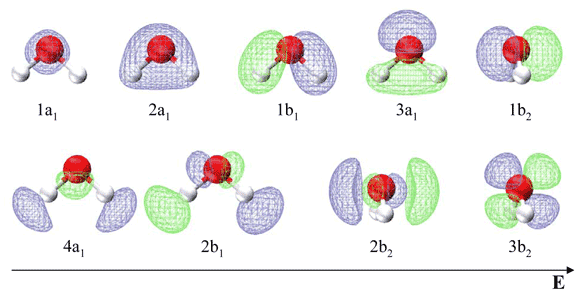
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest Transcribed image text: Part A Use the molecular orbital diagram shown to determine which of the following is most stable Atomic orbitals Molecular orbitals Atomic orbitals TE 2p 2p 22 Tap 25 BCN NA? 82 MacBoo Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic orbitals 3 2p 2P D SS 0, Ne 02 None of the best ... With QM data loaded, VMD can display molecular orbitals, as well as access the calculated energy levels and various other data present in the loaded files [ 4, 3]. In this tutorial output from the GAMESS [2] program will be used to provide input for visual-
H20 molecular orbital diagram. Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. Predicting Reactions Using Frontier Orbitals • Here are two species you have never seen before. Using Frontier Molecular Orbital Theory (FMO Theory), predict the first step of the reaction between these two species. Draw the curved-arrow mechanism that shows how they react, and predict the immediate product of that reaction. Hybradization of the orbitals to [tex]sp^3[/tex] would darastically decrease the repulsion the previous model produces. so the hydrogens would bond to the two avaliable sp3 orbitals. As for the molecular orbital diagram, the lowest energy bonding interaction is a sigma interaction between hydrogen and the afore mentioned hybridization. chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference.
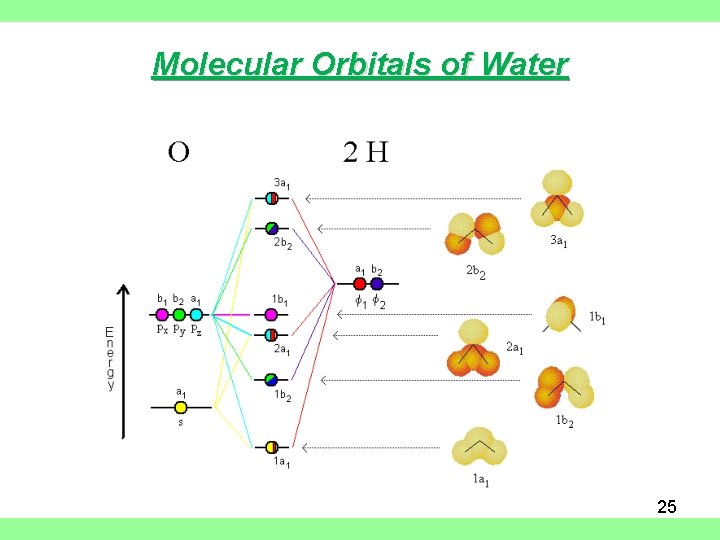
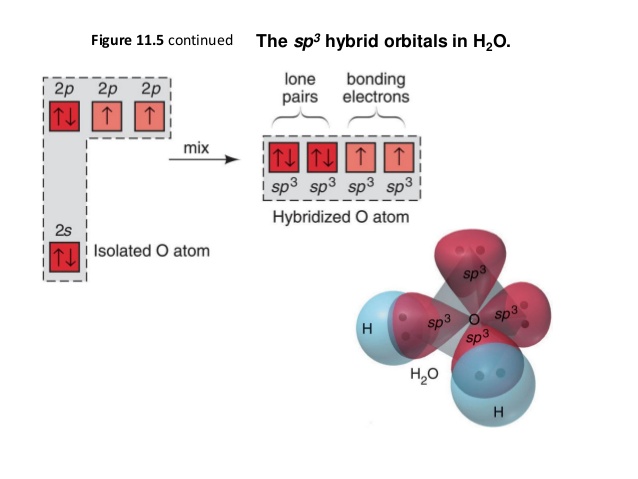
Molecular Orbitals • Themolecularorbital(MO) approachseeks to construct orbitalsfor the bondedsystem. • Approximate wave function solutions are constructed as a Linear Combination of Atomic Orbitals(LCAO). • For diatomicmolecules,AB, these LCAO MOshavethe generalform 1 = a A + b B 2 = a A -b B Construct SALCs and the molecular orbital diagram for H 2 O. Preliminary Steps. Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. Step 2. Identify and count the pendant atoms' valence orbitals. Generate SALCs. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O - WikipediaChemical bonding of H2O - Wikipedia Three 2p orbitals of Oxygen and one 2s orbital are hybridized as there are two pairs of bonding electrons and two lone pairs. And as four orbitals of Oxygen are hybridized, the hybridization of H 2 O is sp3. H2O Molecular Geometry. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons.
Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer. Using group theory to crate a qualitative MO diagram for water H 2 O has a tetrahedral arrangement of molecules or an angular geometry. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion. Additionally, the existing pairs do not lie in the same plane. One pair is below the plane and the other one is above. molecular orbitals and a molecular orbital energy level diagram. These results are given below. You should compare these results with the traditional valence bond (Lewis structure) formulation of the bonding in H20. - 0.15ftP(Hl) + + 0.821Þ(02s) + = + 0.620 ) 2py = 02px 02py--- + 2px = - 2b2 1b 1b2 02.s 01s 0.5011J(02s) + 0.790 ) 2pz - 0.9911J(O )
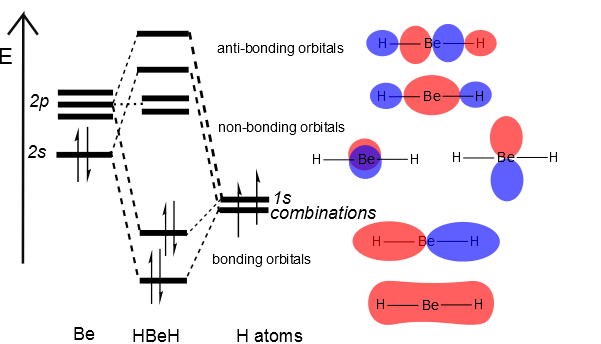
In general, this mixing of n atomic orbitals always generates n molecular orbitals. The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital.
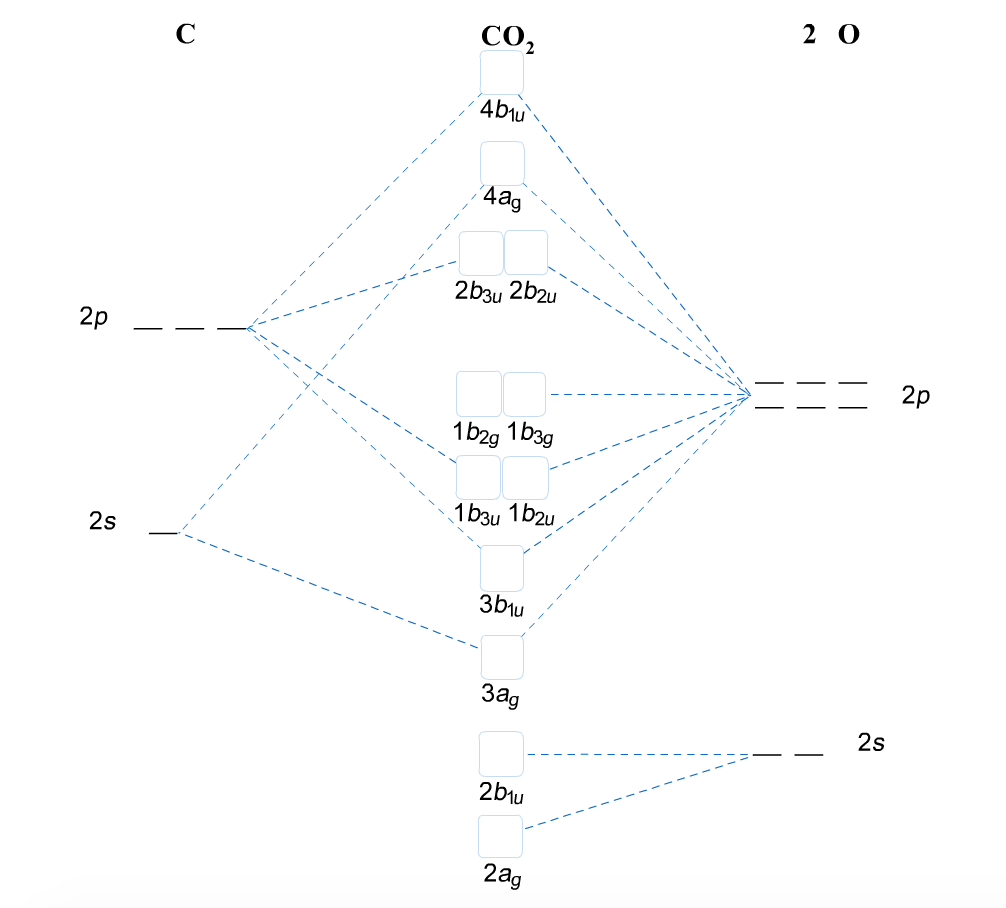
Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion.
इस विडिओ में हमने MOLECULAR ORBITAL DIAGRAM FOR AX2,AX3,AX4 EXAMPLE -H20,NH3,BH3,SiH4 डिस्कस किये हैं | for calculation of bond order in ...
Pj-242 2.5mm Headphone Jack Socket Female Connector For Audio Video 3+3 Smd 6p Wiring Diagram. 29.08.2018.
H20 Molecular Orbital Diagram It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals.
Answer (1 of 7): Oxygen is sp3 hybridised in H2O molecule. Two hybrid orbitals are occupied by lone pairs and two are used in bonding with Hydrogen atoms. Since lone pairs does not contribute to the geometry of a molecule, therefore H2O has an angular geometry. The lone pair-lone pair repulsion i...
I I I I I I I I I I I -5.00 -3.67 -2.33 -1.00 0.33 1.67 3.00 X 4.33 Fig. 2. 3tg Molecular Orbital of [Fe(H20)6 12+, showing predominantly metal contributions. and ligand molecular orbitals are shown in brackets. The sequence of energy levels is similar for each complex, with only two changes in order.
These two orbitals are called the e g orbitals (the symbol actually refers to the symmetry of the orbitals, but we will use it as a convenient name for these two orbitals in an octahedral complex). The other three orbitals, the d xy , d xz , and d yz orbitals, have lobes that point between the ligands and are called the t 2 g orbitals (again ...
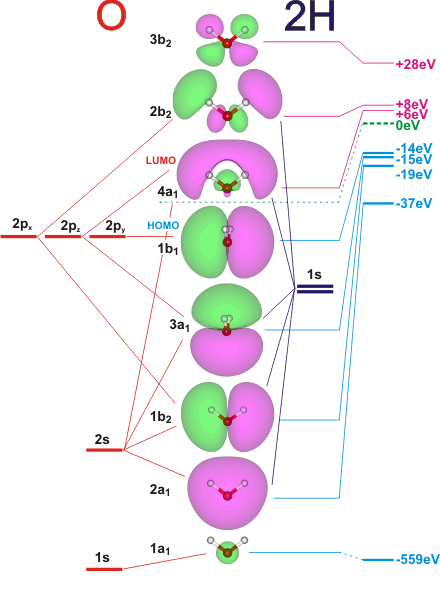
Description. H2O-MO-Diagram.svg. English: MO diagram of water. Vectorized, simplified and corrected from File:Diagramme AH2.png. Quantitative calculations show bonding character in both the 3a 1 and 2a 1 levels. Date. 21 May 2015. Source. Own work.
With QM data loaded, VMD can display molecular orbitals, as well as access the calculated energy levels and various other data present in the loaded files [ 4, 3]. In this tutorial output from the GAMESS [2] program will be used to provide input for visual-
Transcribed image text: Part A Use the molecular orbital diagram shown to determine which of the following is most stable Atomic orbitals Molecular orbitals Atomic orbitals TE 2p 2p 22 Tap 25 BCN NA? 82 MacBoo Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic orbitals 3 2p 2P D SS 0, Ne 02 None of the best ...
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest














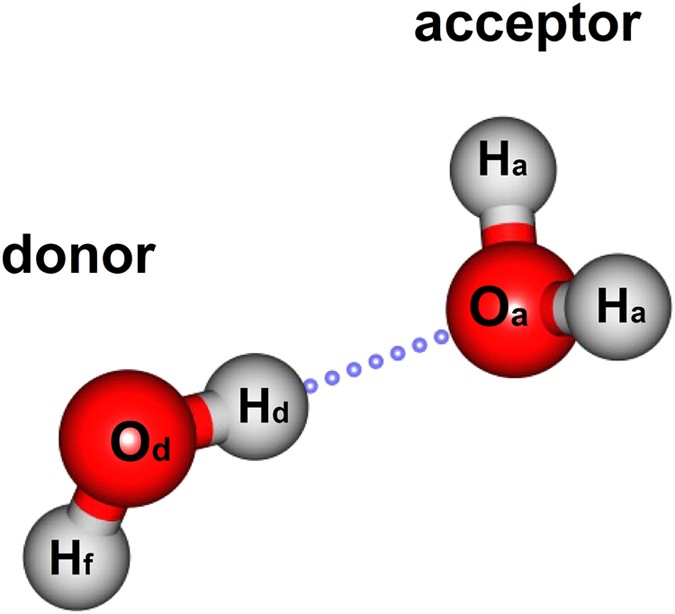

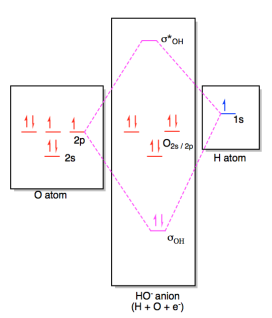
0 Response to "39 h20 molecular orbital diagram"
Post a Comment