39 orbital diagram for cobalt
Electronic configuration of the Cobalt atom. Valence electrons. Orbital diagram.
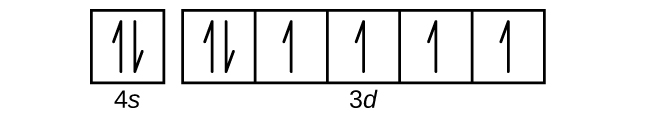
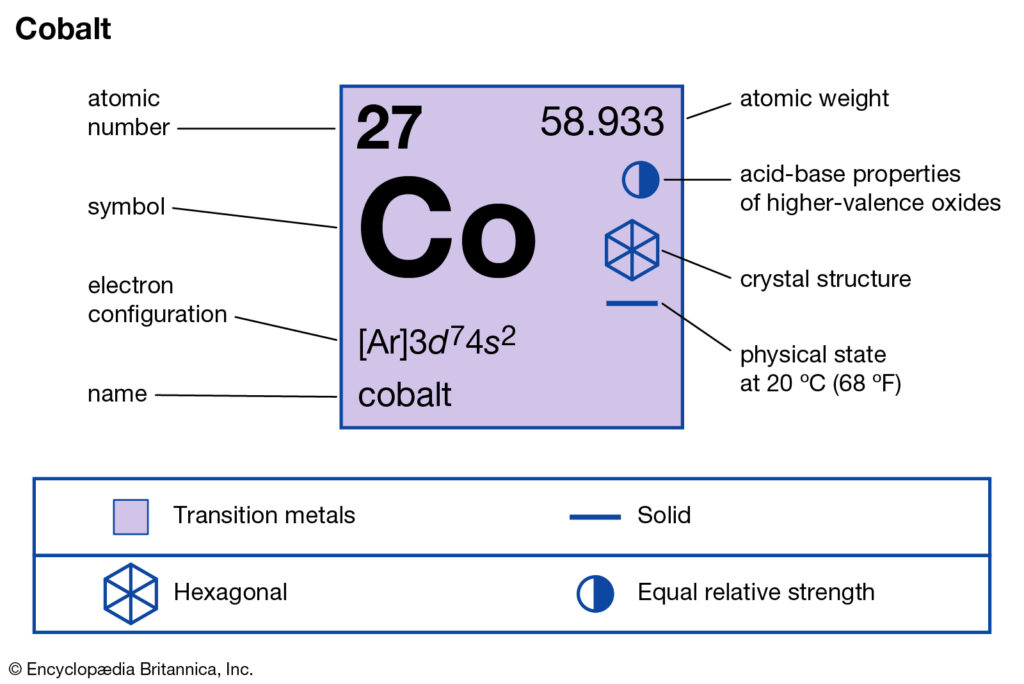
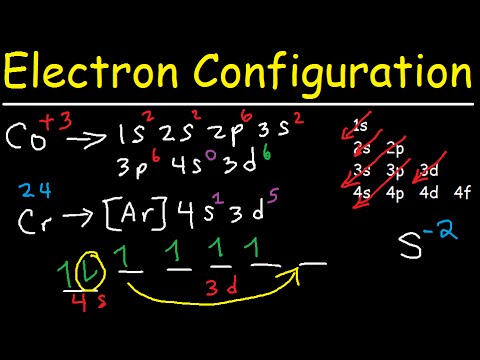
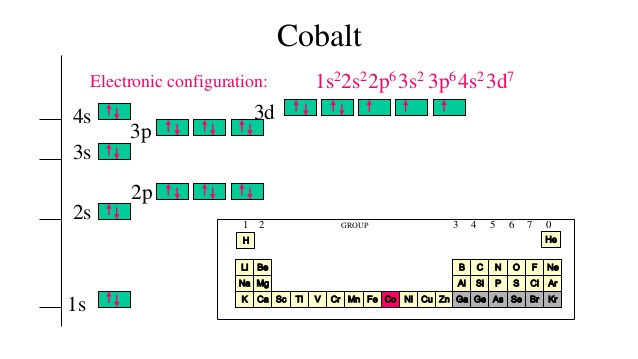
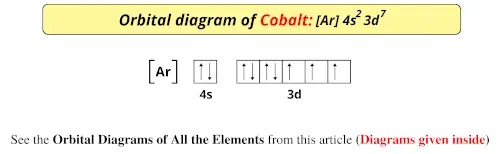
Cobalt (Co) has an atomic mass of 27. Find out about its chemical and ... Electron Configuration, [Ar] 3d7 4s2. 1s2 2s2 2p6 3s2 3p6 3d7 4s2. Orbital Diagram.
Cobalt atoms have 27 electrons and the shell structure is 2.8.15.2. The ground state electron configuration of ground state gaseous neutral cobalt is [Ar].3d7.
Orbital diagram for cobalt
3 Jun 2020 — Answer and Explanation: The electron configuration of cobalt is 1s2 2s2 2p6 3s23p6 3d7 4s2. Cobalt is in the first row of the d-block of ...
Cobalt is part of that elusive iron-cobalt-nickel group within the transition metals. The electron configuration for cobalt is [Ar] 3d7, 4s2.8 answers · 24 votes: As cobalt (Co) has atomic number 27. it's a d-block element.....so electron is get added in ...
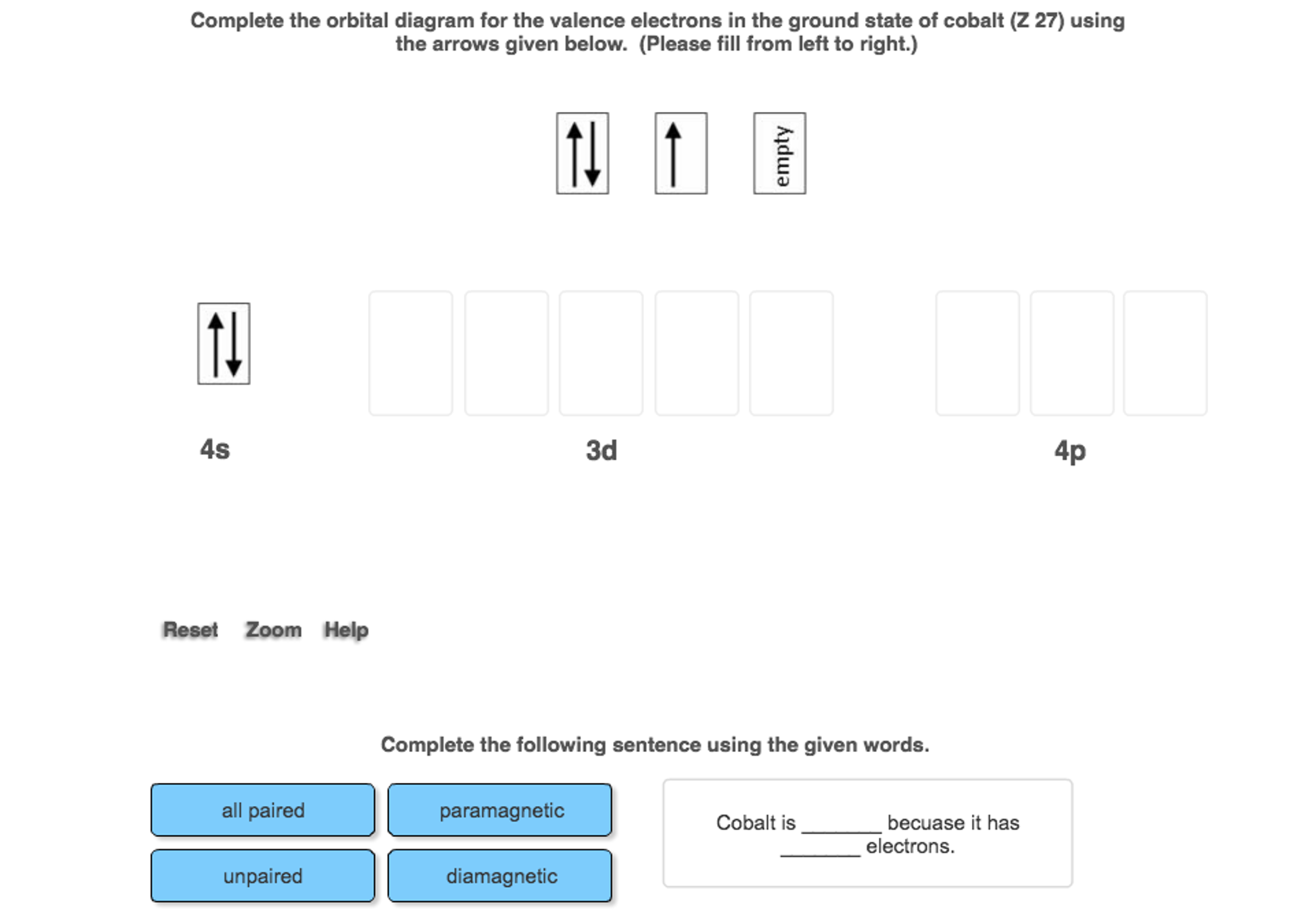
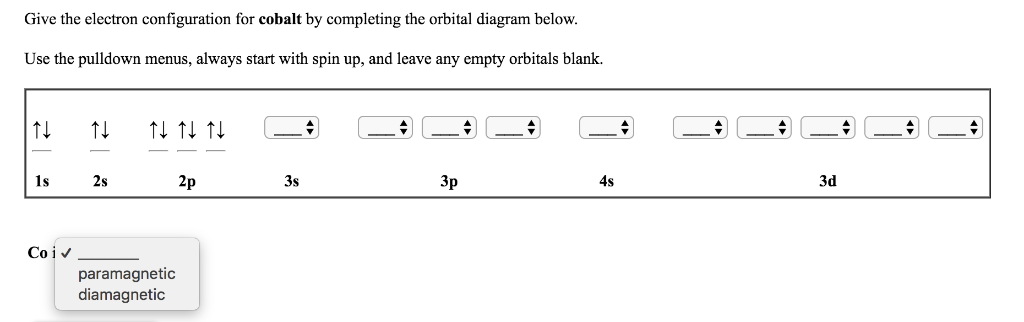
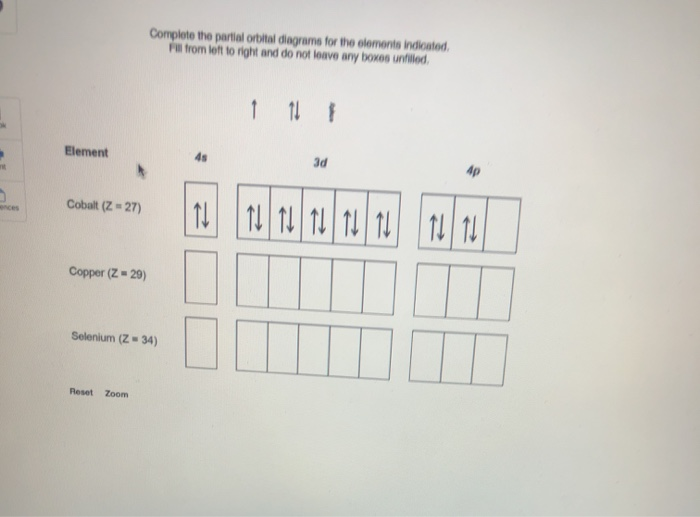
Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms: N; Si; Fe >Te; Mo; Is 1s 2 2s 2 2p 6 the symbol for a macroscopic property or a microscopic property of an element? Explain your answer. Which atom has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 2? Cobalt–60 and iodine–131 …
Orbital diagram for cobalt.
Answer (1 of 9): cobalt has the ground state electron configuration of [Ar] 4s2, 3d7 But when cobalt loses three electrons, it loses them from both the 4s and the 3d. It loses two electrons from the 3d in order to make the 3d half-filled, which has a high degree of stability, and it loses 1 elect...
d7Tanabe-Sugano Diagram E / B ∆o/ B 4F 2G 2Eg 2T1g 2A1g 2T2g 4P 4A 2g 4T 1g (4P) 4T 2g 4T 1g (4F) Complexes with d4-d7 electron counts are special •at small values of ∆o/B the diagram looks similar to the d2diagram •at larger values of ∆o/B, there is a break in the diagram leading to a new ground state electron configuration. d7Tanabe-Sugano Diagram E / B ∆o/ B …
Orbital diagram for cobalt. The purpose of rapid reading assignment is could provide students with specific brief debate about ionic bond characteristics and introduction to my term anion and cation. to label and complete the Venn Diagram identifying the differences and similarities of the Patriots, Loyalists and Undecideds. VSEPR for 4 electron clouds. It contains a pi molecular …
14 Nov 2015 — Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and ...2 answers · The s,p,d,f configuration for cobalt (Co) is 1s22s22p63s23p64s23d7, determined by the position ...
26 Jan 2021 — Full electron configuration can be defined as 27 electrons distribution in 4 shells of Co element. There are 2, 8, 15, 2 elements present in the ...
The diagram shows the arrangement of the d electrons in a Cu 2+ ion before and after six water molecules bond with it. Whenever 6 ligands are arranged around a transition metal ion, the d orbitals are always split into 2 groups in this way - 2 with a higher energy than the other 3. The size of the energy gap between them (shown by the blue arrows on the diagram) varies with …
Cobalt(II) hexahydrate. Another example is [Co(H 2 O) 6] 2+. Note that the ligand is the same as the last example. Here the cobalt ion has the oxidation state of +2, and it is a d 7 ion. From the high-spin (left) side of the d 7 Tanabe–Sugano diagram, …
Lithium Cobalt Oxide – LiCoO 2; Lithium Manganese Oxide – LiMn 2 O 4; Anode (Negative electrode) Material Examples . Graphite anode; Lithium titanate-Li 4 Ti 5 O 12; Silicon Anode; LISICON – Solid Electrolyte example; Structure and Bonding. Atomic Orbitals. s-orbitals; p-orbitals; 3p-orbitals; 3d-orbitals; 4f-orbitals; Compare shape and size of 1s, 2s and 2p …
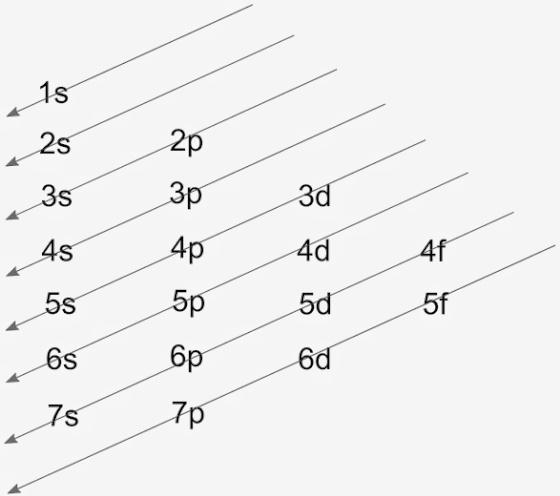
Cobalt : atomic number (Z) = 27 (d block element) Aufbau Principle: 2 electrons occupy the completed first energy level (K shell), 8 electrons occupy the completed second energy level (L shell), and 8 electrons occupy the s and p orbitals of the third energy level (M shell). Note that we need to place 9 electrons into 6 orbitals of very similar energy (4s, 3d xy, 3d xz, 3d yz, 3d x 2 …
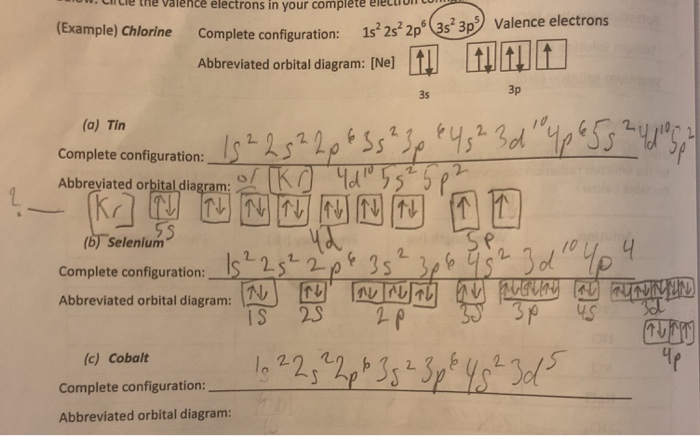
01/11/2021 · Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: …
1 answerGiven that the atomic number (Z) is 27, we know the neutral cobalt atom will have 27 electrons. These electrons are distributed into orbitals using the ...
05/06/2017 · When observing Cobalt 3+, we know that Cobalt must lose three electrons. The first two to go are from the 4s orbital and Cobalt becomes:[Ar]4s 0 3d 7. Then, the next electron leaves the 3d orbital and the configuration becomes: [Ar]4s 0 3d 6. Thus, we can see that there are six electrons that need to be apportioned to Crystal Field Diagrams ...








![6) cobalt [Ar] 4s 2 3d 7](https://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf.png)

![SolveLancer Test]Which of the following i... - Organic Chemistry](https://media.kunduz.com/media/question/raw/20210619161606882659-2869725.jpg?h=512)





/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)







0 Response to "39 orbital diagram for cobalt"
Post a Comment