40 orbital filling diagram for oxygen
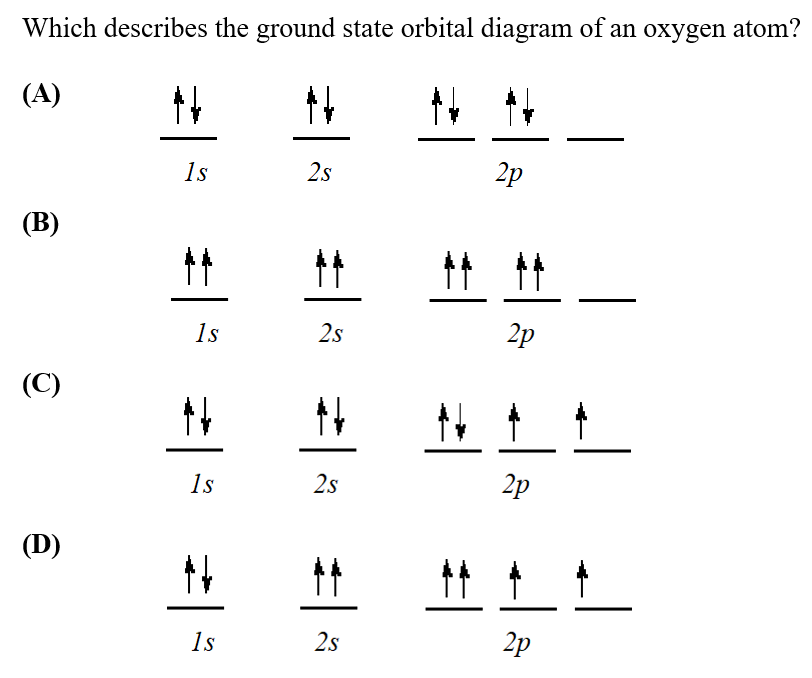
0.s Which orbital-filling diagram represents the ground state of oxygen? O [He] 11 2s 2p 1 [He] 们个个 2p 2 2s โต | 44 | O [He] 11 111 2p 3 2s O [He] ] 们 2s N 4 2p 5 Previous
Which orbital-filling diagram represents the ground state of oxygen? A. [ [He] 신 N 2p 치어 B. [He] 신 소 소 2p 25 C. [He] 个个 2s 신 소 소 2p D. [He] _ 28 솨 신 소 2p 치 ; Question: Which orbital-filling diagram represents the ground state of oxygen? A.
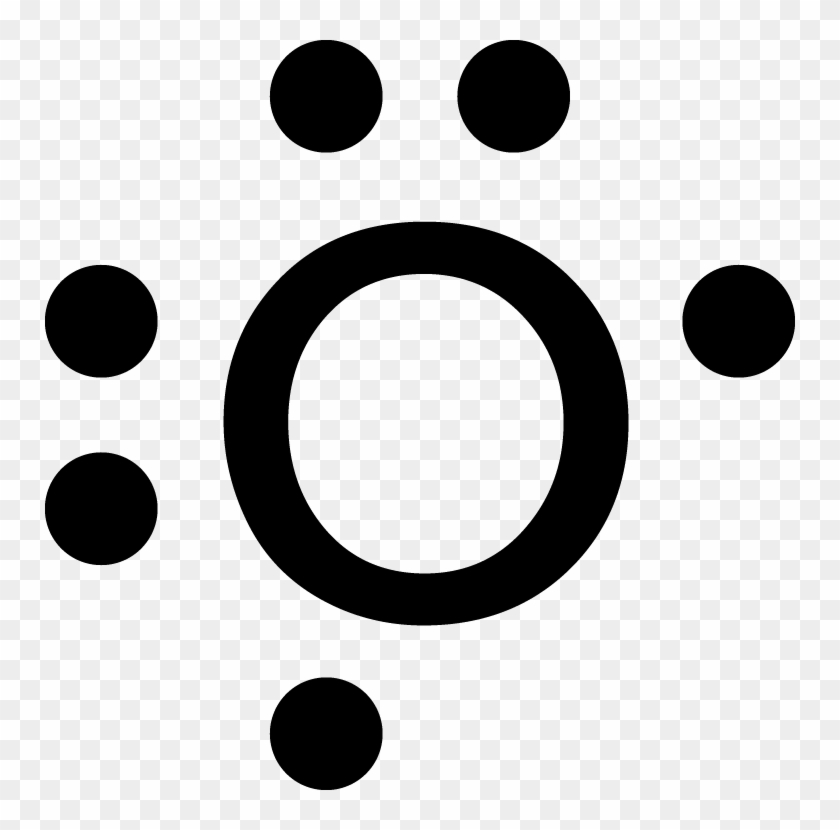
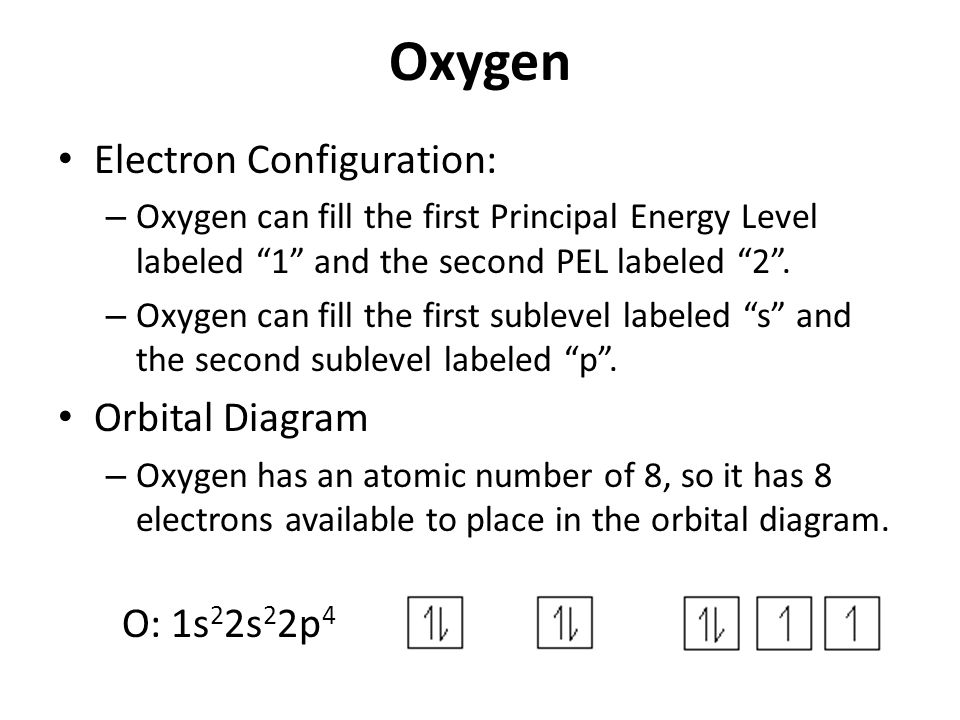
2. Electron Configuration (quicker to draw than orbital filling diagrams) Ex. O2 1s2 2s2 2p4. 3. Electron Dot shows only the valence (outer energy level) electrons. . Ex. Oxygen atom . O :. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram
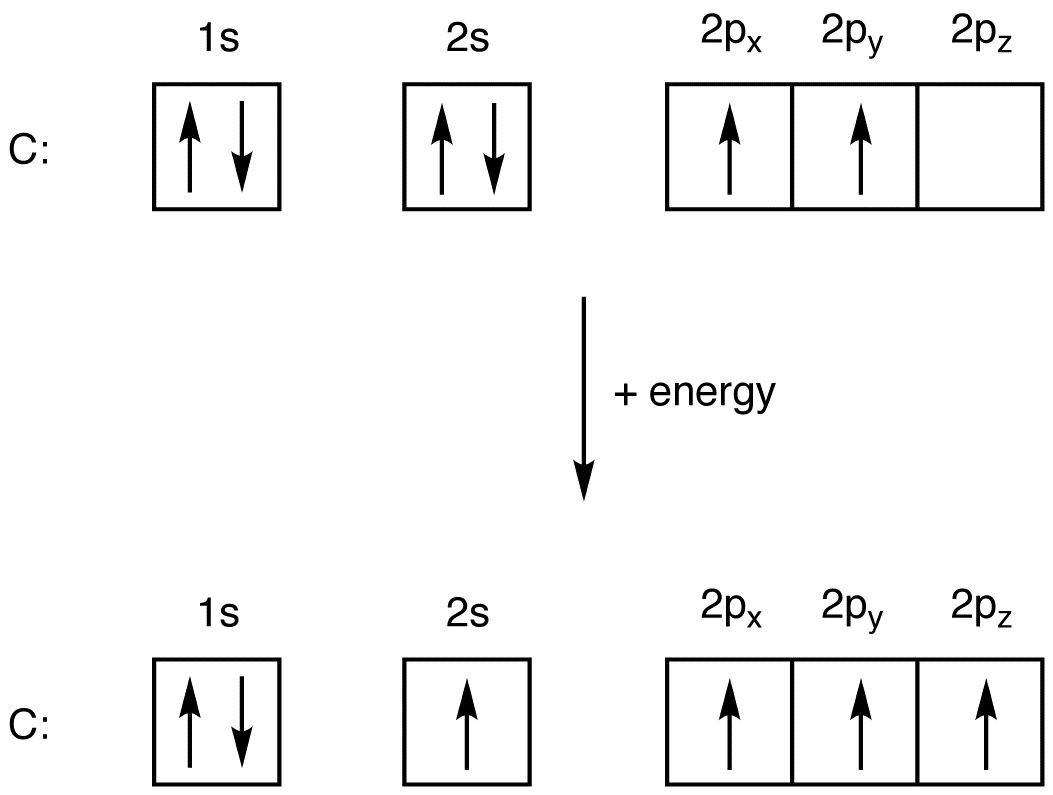
Orbital filling diagram for oxygen
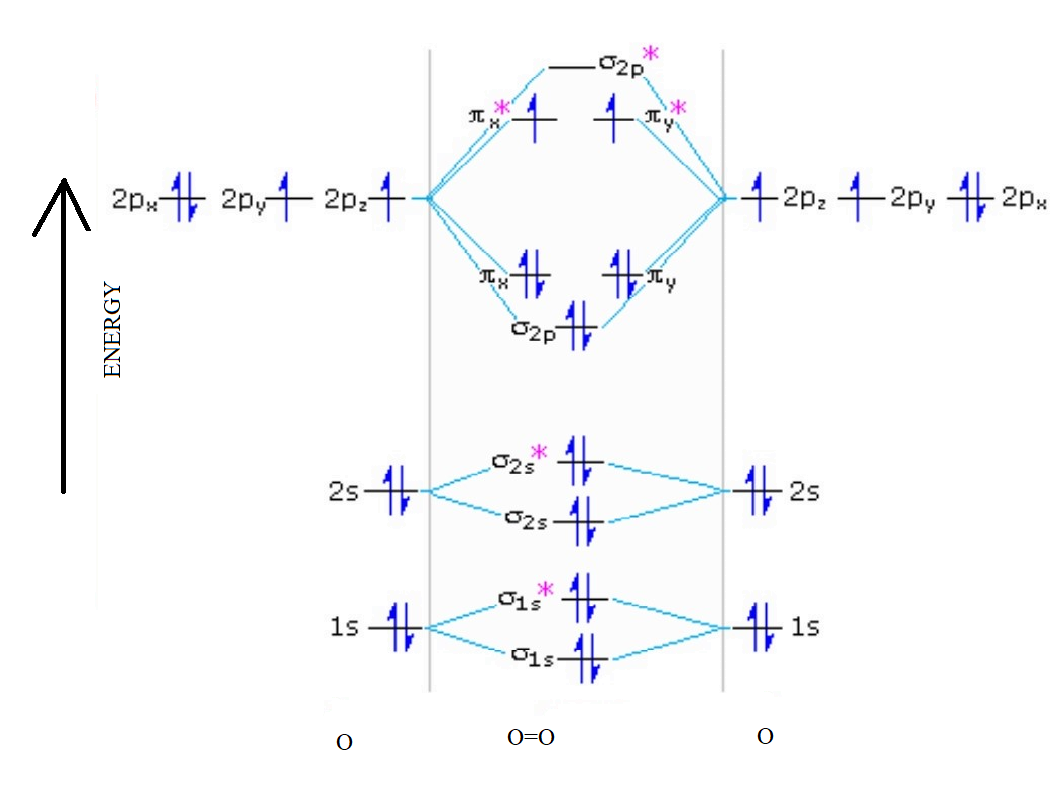
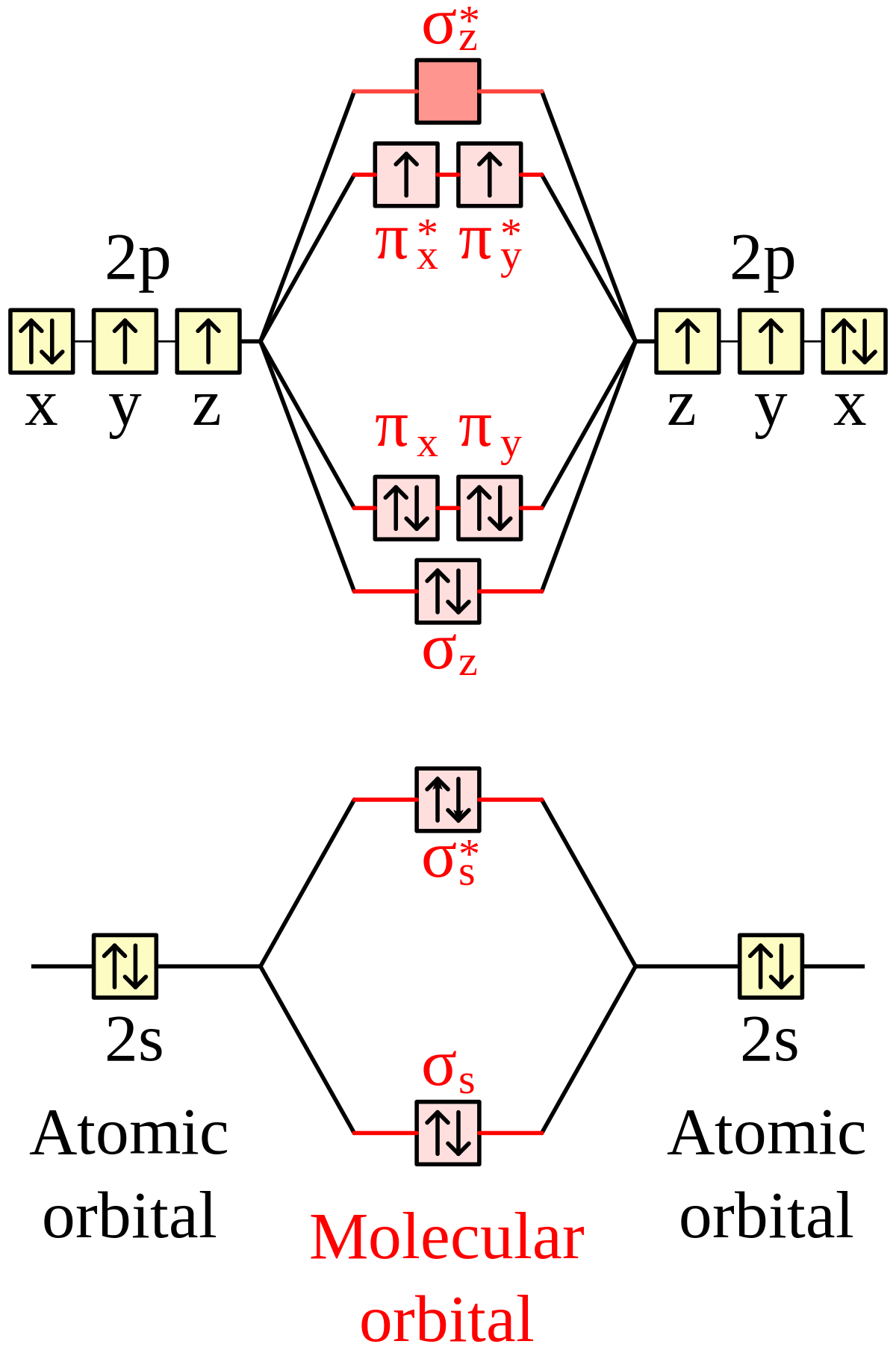
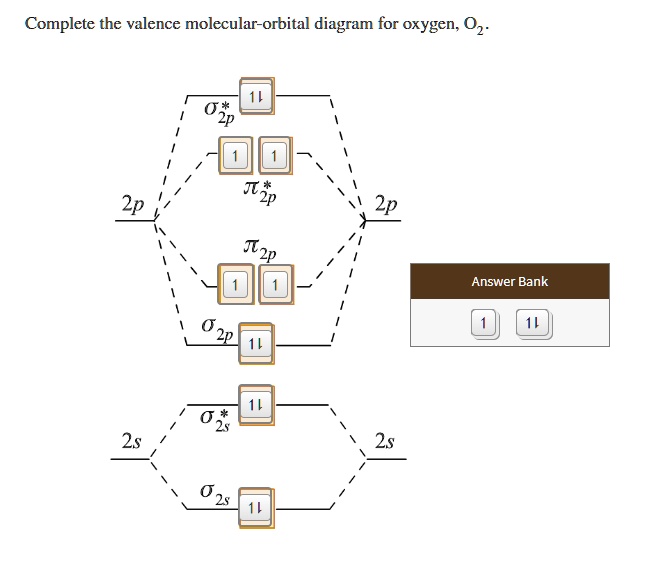
The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for O 2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules ...
Molecular orbital energy level diagrams -Hydrogen, Hypothetical, Nitrogen, Oxygen. The filling of molecular orbitals is governed by the following principles. (i)Aufbau principle (ii)Pauli's exclusion principle and (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules.
The atomic orbital sets of similar energy are grouped into a series of seven shells, which is equal to the number of periods in the periodic table. Complete the energy-level diagram by filling the number of electrons in each orbital set within a shell, and also give the total number of electrons in each shell.
Orbital filling diagram for oxygen.
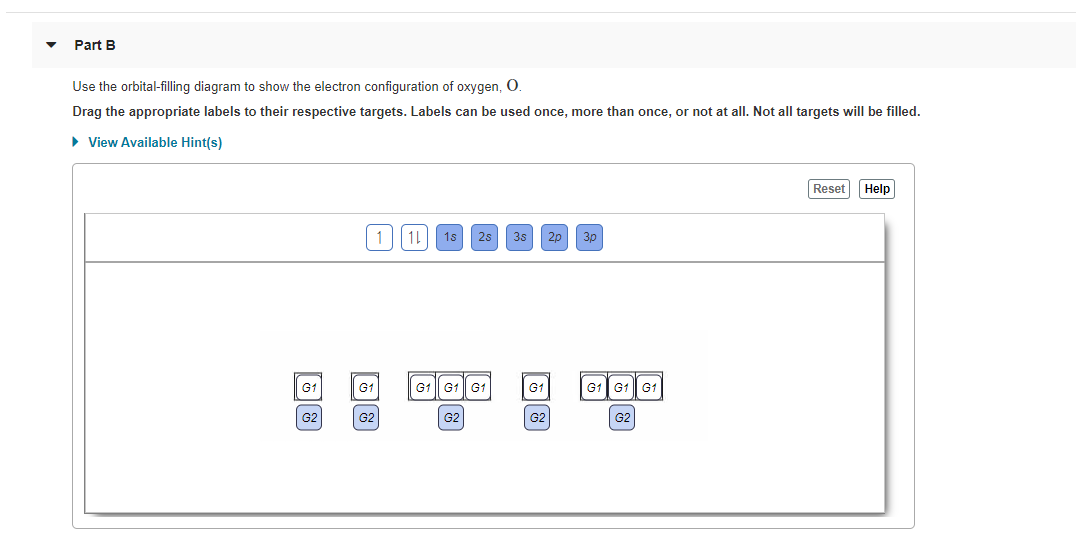
Use the orbital-filling diagram to show the electron configuration of oxygen, O. Use the orbital-filling diagram to show the electron configuration of gallium, Ga. Which of the following is the correct electron configuration for nitrogen? 1s 2s2 3p3 1s 22s 22p 3 1s 22s 21p 3
Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
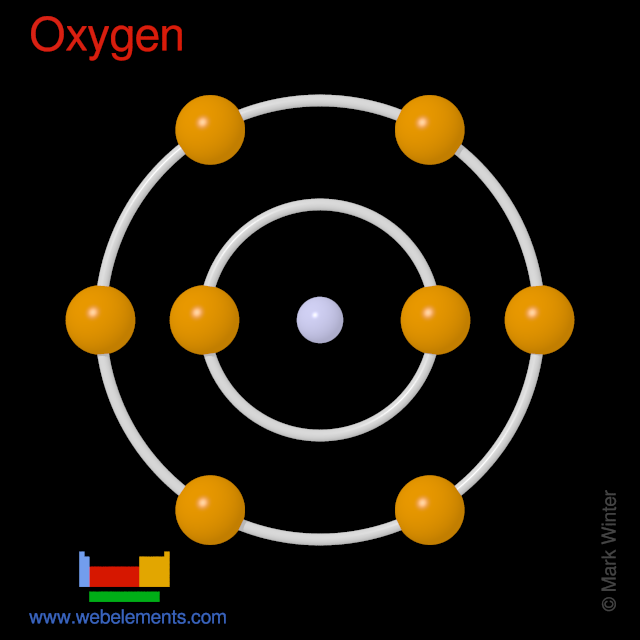
The atomic number of Oxygen is 8 .So, it has 8 valence electrons. The Electronic configuration of oxygen can be written as 1s22s22p4 The atomic number …. View the full answer. Transcribed image text: Fill in the orbital energy diagram for the oxygen atom. 2p E 2s ls Fill in the orbital energy diagram for magnesium. 3p 3s E 2p 2s Is.
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. 6.5K views ·.4 answers · 8 votes: Four electrons in the lowest levels, sigma-1 and sigma*-2 (bonding and antibonding), two in ...
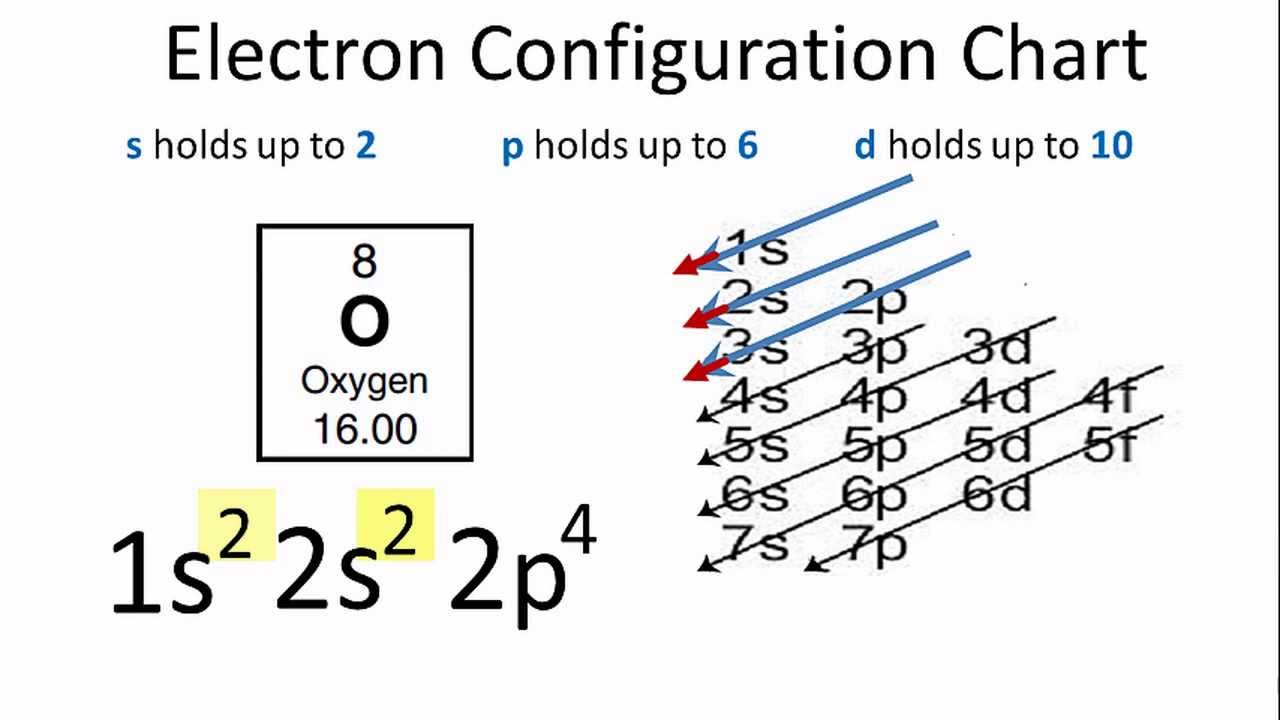
Oxygen (O) Electron Configuration with Full Orbital Diagram Oxygen electron configuration is 1s 2 2s 2 2p 4. The period of oxygen is 2 and it is a p-block element.
You are watching: Use the orbital-filling diagram to show the electron configuration of phosphorus, p. Electron Configuration. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons.
Chemistry Q&A Library Fill in the orbital energy diagram for the lithium ion. 2p- E 2s- 1s- Fill in the orbital energy diagram for the lithium ion. 2p- E 2s- 1s- close
Sep 15, 2016 — The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram.1 answer · http://www.thestudentroom.co.uk/showthread.php?t=3933809&page=30 Explanation: The electron configuration for oxygen is: 1s22s22p4 This video will ...
The two unhybridized p orbitals on carbon form p bonds to the oxygen atoms. The energy diagram for carbon in CO 2 is shown below. What is the hybridization of oxygen in CO 2. Each oxygen has two lone pairs and forms one s bond and one p bond. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond.
By predicting the shapes of the MOs that result from each combination, you will fill in the missing MOs from our Molecular Orbital Diagram for oxygen. To begin, use the list of possible combinations menu to the right of the MO diagram to view all of the possible MOs for oxygen and match them to each individual case that is provided on the right.
Use orbital filling diagrams to describe the locations of electrons in an atom. ... Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 7.7.15. Figure 7.7.15. The molecular orbital energy diagram for O 2 predicts two unpaired electrons. We calculate the bond order as [latex]{\text{O}}_{2}=\dfrac{\left(10-6\right ...
Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
1. Orbital Fillinq Diaaram 02 Ex. 2. Electron Configuration 02 (gives the most information) Is 2s (quicker to draw than orbital filling diagrams) 1 s2 2s2 2p4 3. Electron Dot shows only the valence (outer energy level) electrons EX. Oxygen atom 1 . Write orbital filling diagrams, electron configurations, and electron dot diagrams
So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. ... we know that Oxygen always forms 2- ions when it makes an ion. ... Orbital Diagrams.
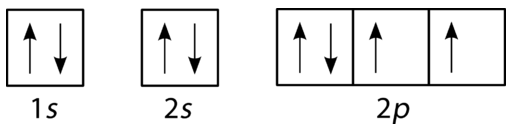
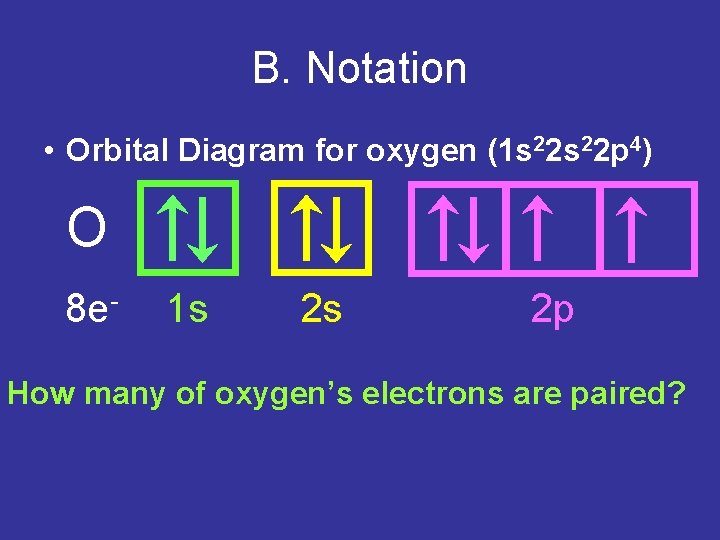
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be ...
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
To fill the diagram, first, we fill each side of the diagram with the electrons according to nitrogen's electron configuration - [He]2s 2 2p 3. Next, we fill the middle section with the molecular orbital's electron configuration using Hund's Rules, just as we do with atomic orbitals. We fill each shell with two electrons before moving to ...
Orbital filling diagram for oxygen. One orbital has a pair of electron while the other two has an unpaired electron. Each horizontal line represents one orbital that can hold two electrons. Energy levels of molecular orbital has determined practically by spectroscopic studies.




















0 Response to "40 orbital filling diagram for oxygen"
Post a Comment