40 periodic table bohr diagram
Bohr Diagrams Find your element on the periodic table. Determine the number of electrons it is the same as the atomic number.This is how many electrons you will draw. Bohr Diagrams Find out which period your element is in. Elements in the 1st period have one energy level.Elements in the 2nd period have two energy levels, and so on.
In this article, we'll focus on one of those models: the Bohr model, which was first proposed by Niehls Bohr in 1913. In this article, we'll explain what the Bohr atomic model is, give a Bohr diagram for the first element, as well as provide information for the 20 elements on the periodic table, and explain how the Bohr model is used.
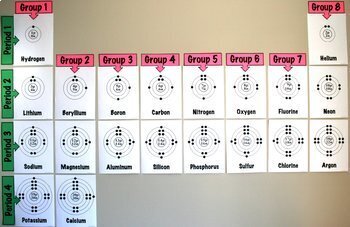
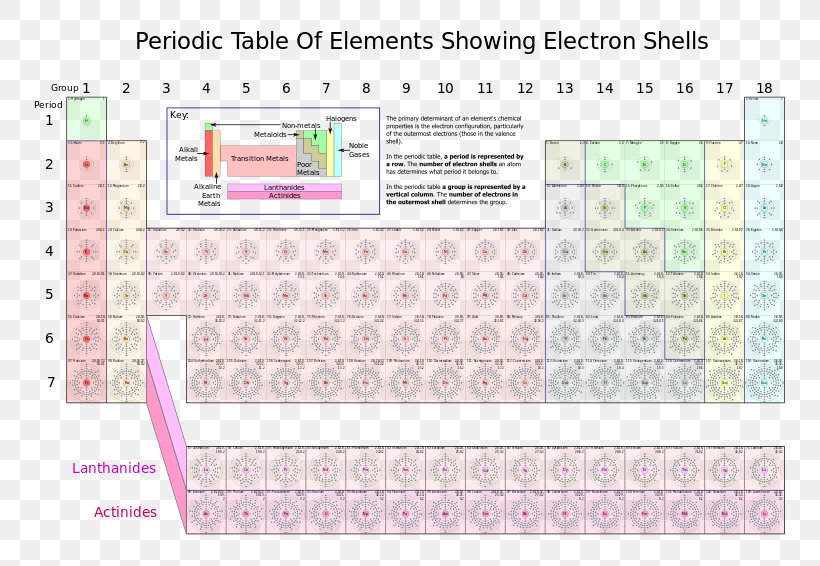
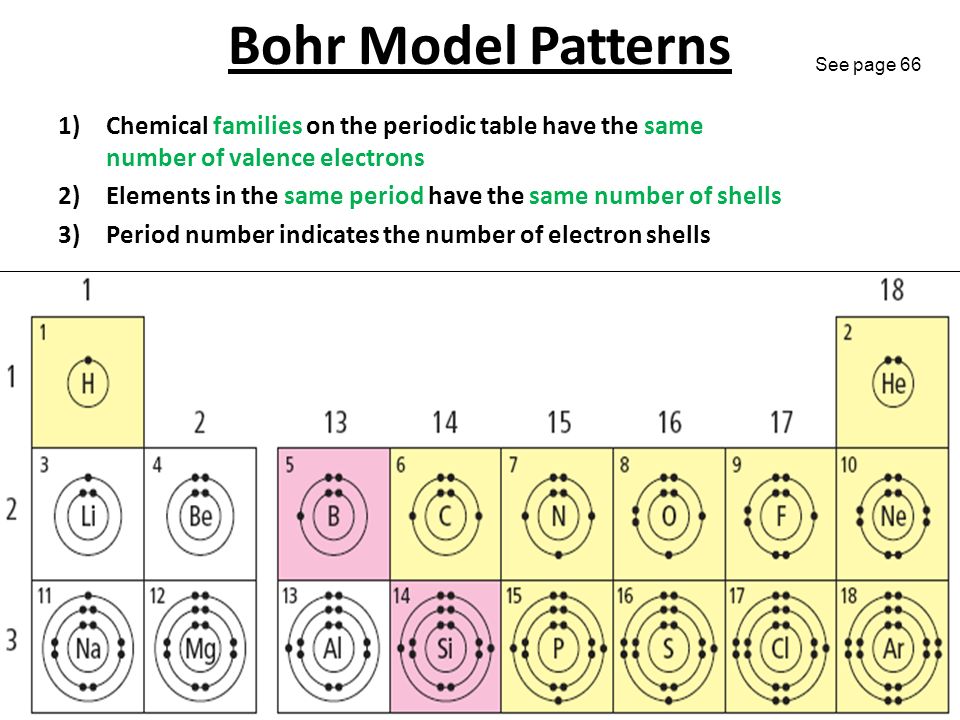
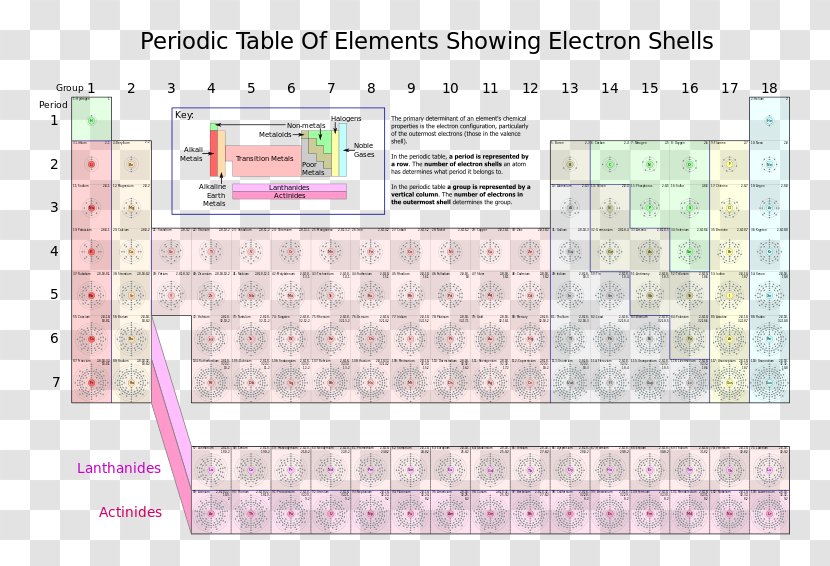
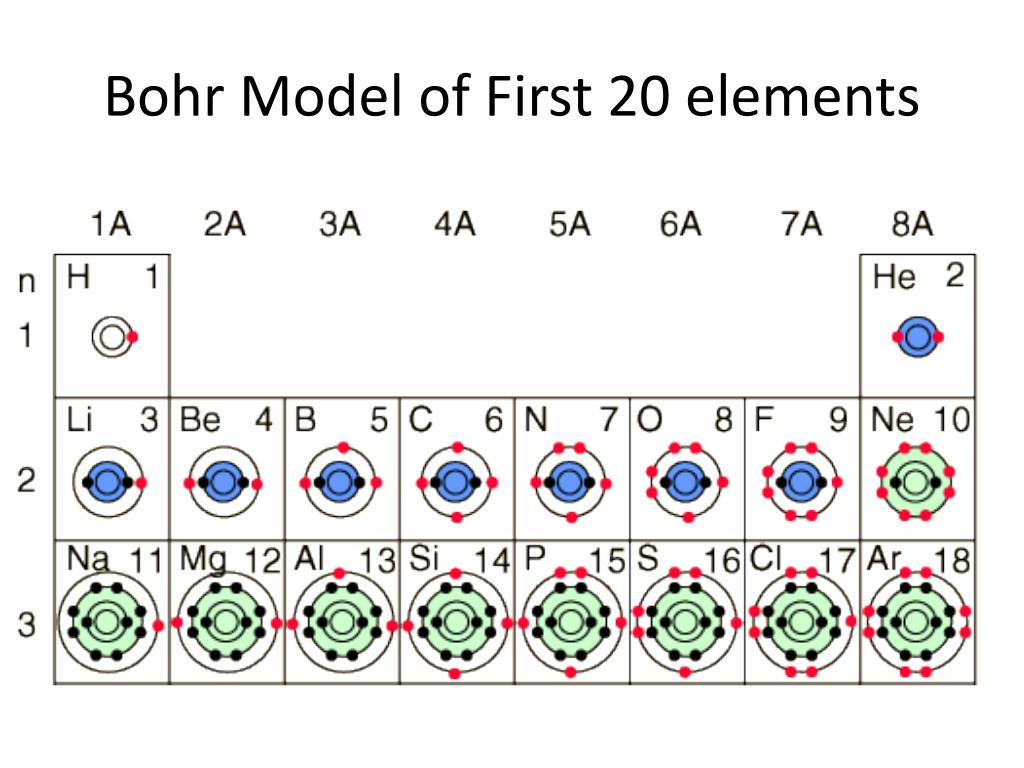
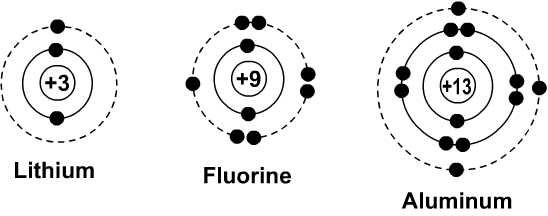
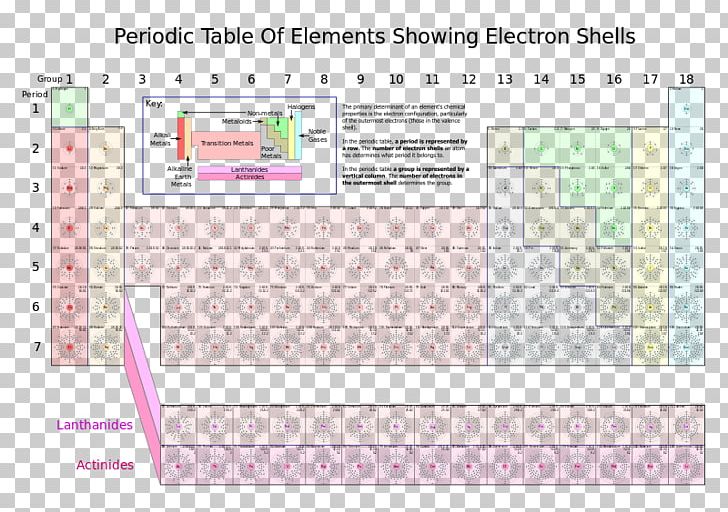
you move down the table the more shells you added to the diagram. H = 1 shell, Li = 2 shells, K = 3 shells. 䡦Moving left to right on the periodic table adds valence electrons to the shells of that row. Na has 1 valence e-, Mg has 2 valence e-, Al has 3 valence e-, etc.
Periodic table bohr diagram
Bohr Diagrams and Lewis Dot Structures ... The # of Valence e- an atom has is dictated by the Group the Element is in Groups are the Vertical Columns on the Periodic Table!!! It's So Simple!!!! Practice: The First Two Are Tricky so pay attention Practice: All Elements in the first group have only 1 Valence e- Practice: All Elements in the ...
Bohr Model of all Elements (Diagrams + Chart) November 1, 2021 March 7, 2021 by Admin. Bohr model of all Elements is mentioned in the chart below. ... Periodic table with Electronegativity values (labeled image) Periodic table with Valence Electrons Labeled (7 HD Images)
Learn bohr models periodic table with free interactive flashcards. Choose from 500 different sets of bohr models periodic table flashcards on Quizlet.
Periodic table bohr diagram.
Bohr Diagrams 1) Find the element on the periodic table. 2) Determine the number of electrons--it is the same as the atomic number. 3) This is how many electrons you will draw. Bohr Model Diagrams • Find out which period (row) your element is in. • Elements in the 1st
Bohr Diagrams 1) Find your element on the periodic table. 2) Determine the number of electrons -it is the same as the atomic number. 3) This is how many electrons you will draw. Bohr Diagrams •Find out which period (row) your element is in. •Elements in the 1st period have one energy
Welcome to Gr. 10 Science! In this video series, we'll be exploring the topics of nomenclature & chemical bonds. Make sure you complete your homework each ni...
So how do we draw a Bohr model of… say…Sodium? Start with a nucleus, and put in how many protons and neutrons are in sodium (look at your periodic table) Next place some orbits around it P:11 N:12 P:11 N:12 Next figure out how many e- are in sodium (look at your periodic table). Place them in dots in the orbits P:11 N:12 Remember!! 2 max in 1 st
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
bohr diagrams show electrons orbiting the nucleus of an atom in the bohr model, electrons are pictured as traveling in circles at different shells, each element, when electrically neutral, has a number of electrons for example, the 1n shell represents the first energy level located closest to the nucleus.now offering rare physics books for sale …
1) Review topics to be covered on the Matter and Chemical Bonding Test on Thursday: . -the periodic table (know some names of groups, reactivity) -ionic compounds versus molecular compounds (what makes them different) -drawing lewis-dot diagrams for elements, compounds. -bohr-rutherford diagrams.
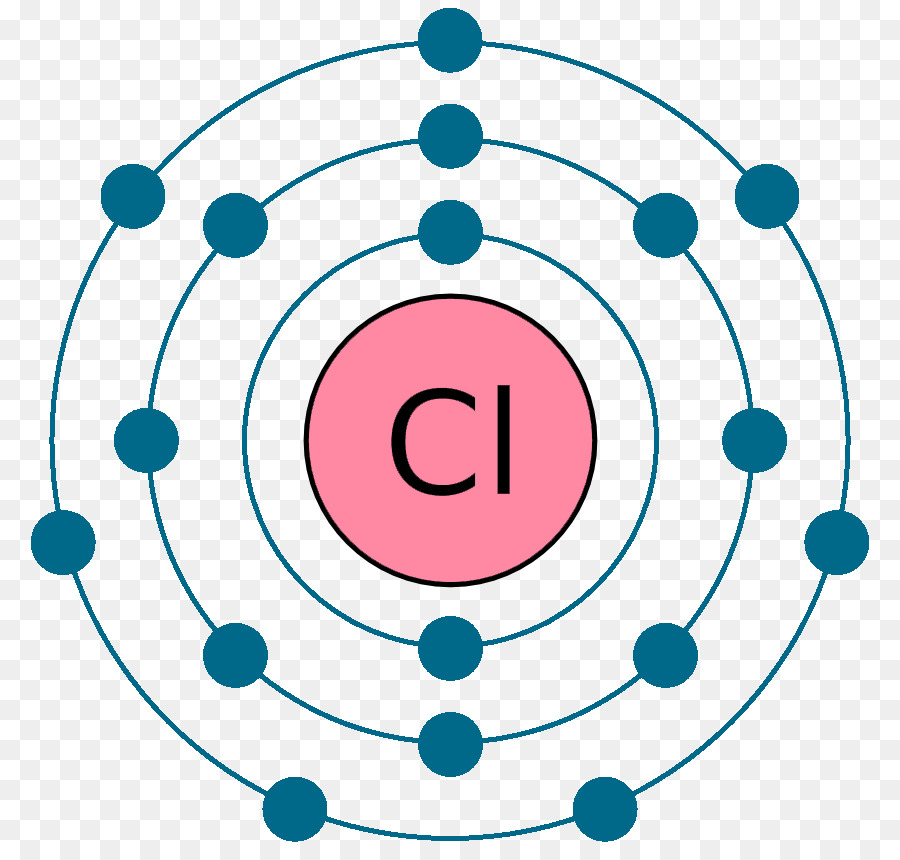
The Bohr Diagrams. The dots that are red are the elements valence electrons. The valence electrons are the electrons in the outer energy level. There can only be up to 8. But when the outer one is not filled, the element is not happy because all it wants is to have a full valence shell. These are the Lewis Dot Structures of the elements.
bohr model in atomic physics the rutherford-bohr model or bohr model or bohr diagram introduced by niels bohr and ernest rutherford in 1913 depicts the atom as a chemical elements an interactive periodic table of an up to date periodic table with detailed but easy to understand information
Drawing a Bohr Diagram Write the element's at the TOP left and the in the atom. Write the number of protons 5. with the at the BOTTOM left the number of (p+) and neutrons (no) as the atom have? electrons. Make your electrons : How many electrons does the the K shell.
Bohr Diagrams • A Bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. • Named in honour of _____, a Danish physicist who developed several models for showing the arrangement of electrons in atoms. • There are three main background questions to explore before we start drawing Bohr diagrams.
Bohr-Rutherford Diagrams We have looked at atomic models and the structure of atoms. Today we will practice drawing those models for the elements on the periodic table. Remember that protons and...
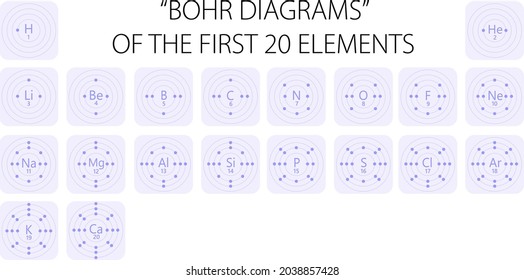
The second page, Bohr-Rutherford Review - Crack the Puzzle, has students practice drawing Bohr-Rutherford diagrams for the first twenty elements. Students match each element's number of protons, neutrons and electrons with a letter to solve the riddle at the bottom of the page.
These cards depict Bohr Diagrams for elements #1-20 of the periodic table. This activity is great to use once your students understand that the "identity" of an atom can be determined by its number of protons, and that in a neutral atom the protons equal the electrons.
An up-to-date periodic table with detailed but easy to understand information. Home About This Site Comments Help Links Window Version. Show Table With: Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: Alkali Metals Alkaline Earth Metals ...
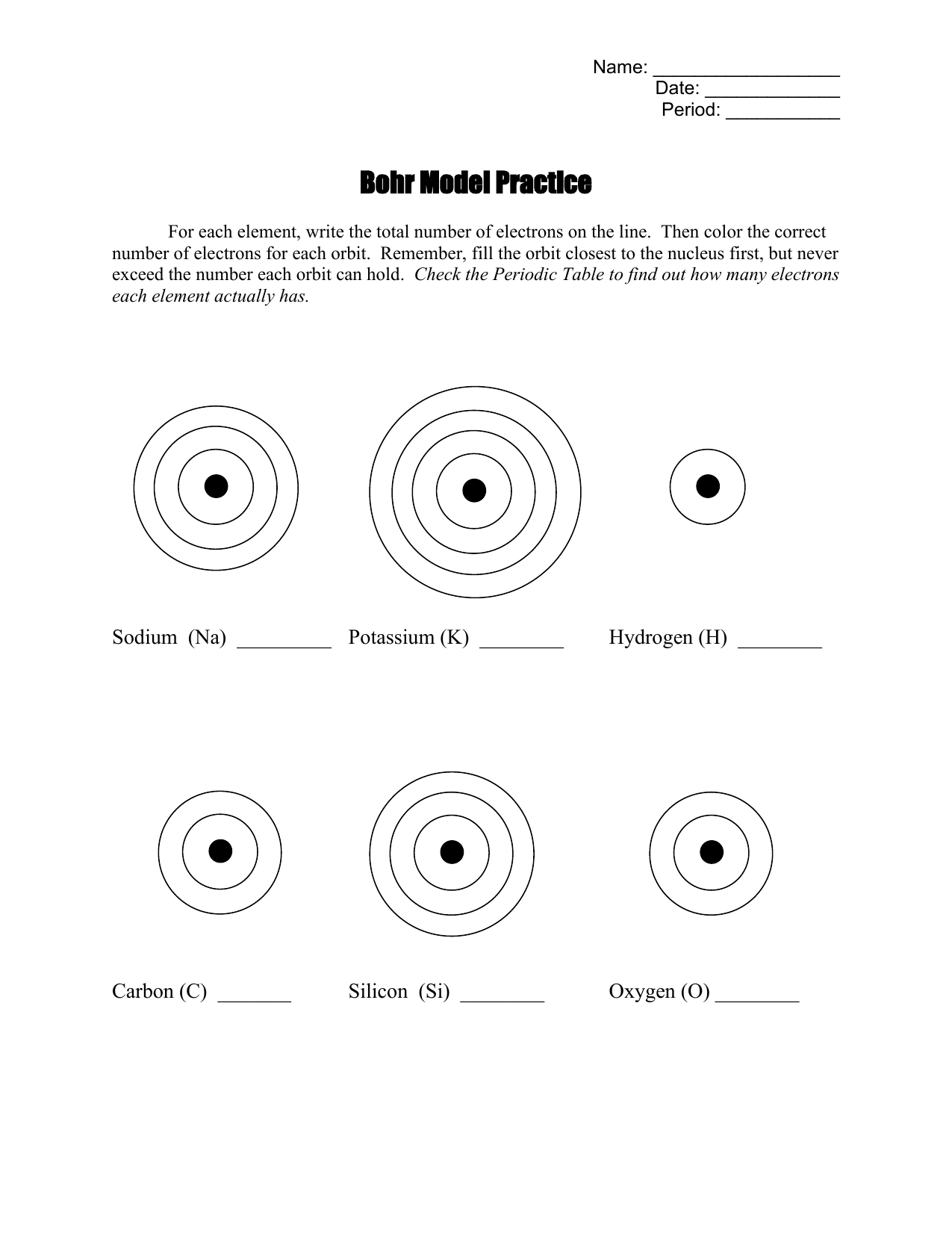
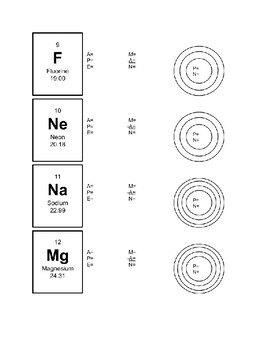
Bohr Model Practice 1. Carbon , 4. Chlorine 7. Aluminum 10. N'ì NAME BLOCK 3. Oxygen 6. Neon 9. Helium 12. Beryllium 2. Hydrogen 009 5. Sodium 1 8. Magnesium 11. Silicon Science 9 Chapter 2 — Elements and the Periodic Table Identify the elements whose Bohr model diagrams are shown below. Write the names of the elements in the spaces provided.
Bohr model and lewis dot diagram worksheet answers periodic table worksheets doc new blank bohr model worksheet fill in for first 20 elements and lewis dot diagram worksheet answers collection of structure with diagrams bohr and lewis models as well some naming review we marked went along the key is below if you didn t finish it homework.
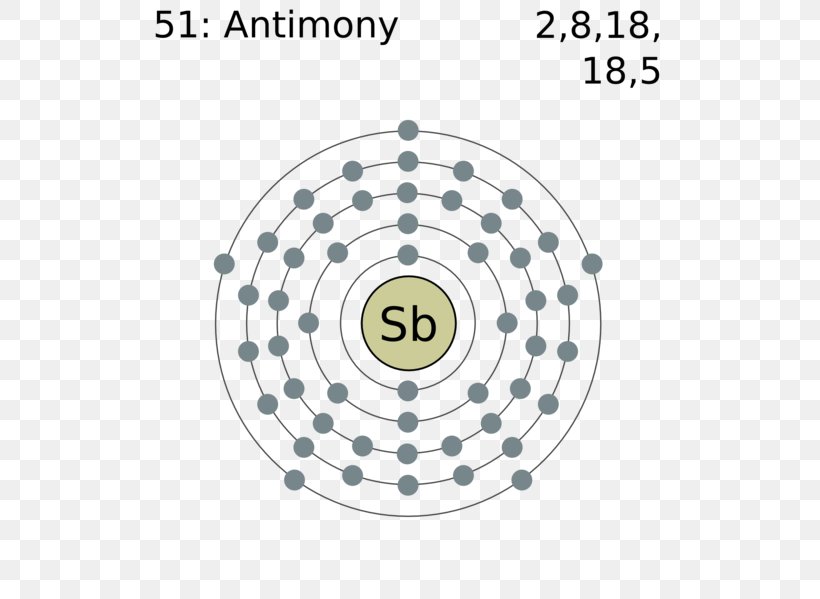
Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.• The 2nd shell can hold up to 8 electrons.
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon here) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron ...
Periodic Table Basics Step 1: Complete the squares for each element by adding the atomic number, name, and atomic mass. ... and electrons in each element. Step 3: Create a Bohr diagram for each element. Step 4: Draw the Lewis Structure for each element. Step 5: Use the following colors to shade in the square for each element. You should
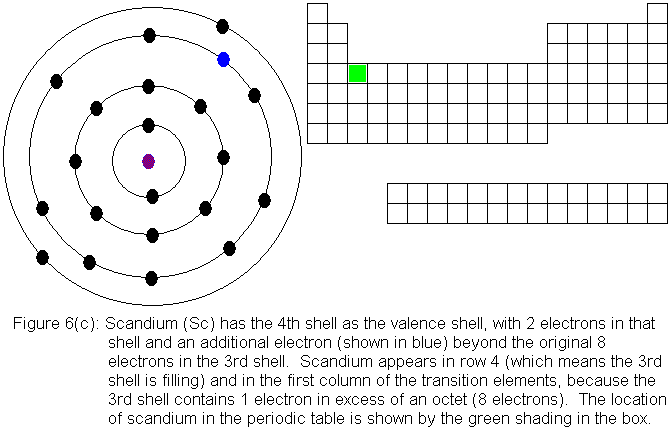
Thus, the Bohr model for heavier atoms described electron shells. The model explained some of the atomic properties of heavier atoms, which had never been reproduced before. For example, the shell model explained why atoms got smaller moving across a period (row) of the periodic table, even though they had more protons and electrons.
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Sodium atom with some simple steps. ... So, the Sodium atom belongs to the 3rd Period in the periodic table, hence, the number of electron shells for the Bohr model of Sodium will be 3 (K-shell, L-shell, and M-shell).
#5: Bohr Diagram Manipulatives Activity. You will really hear the Eurekas! in your classroom during this activity. When students construct the Periodic Table in this Bohr Diagrams Atom Manipulatives Activity, they will see the patterns that exist in the table without you saying anything. This activity involves simple graphics of the atoms of ...





![How to-bohr-diagram[1]](https://image.slidesharecdn.com/how-to-bohr-diagram1-121113154654-phpapp01/95/how-tobohrdiagram1-14-638.jpg?cb=1352821646)














0 Response to "40 periodic table bohr diagram"
Post a Comment