37 potential energy diagram with catalyst
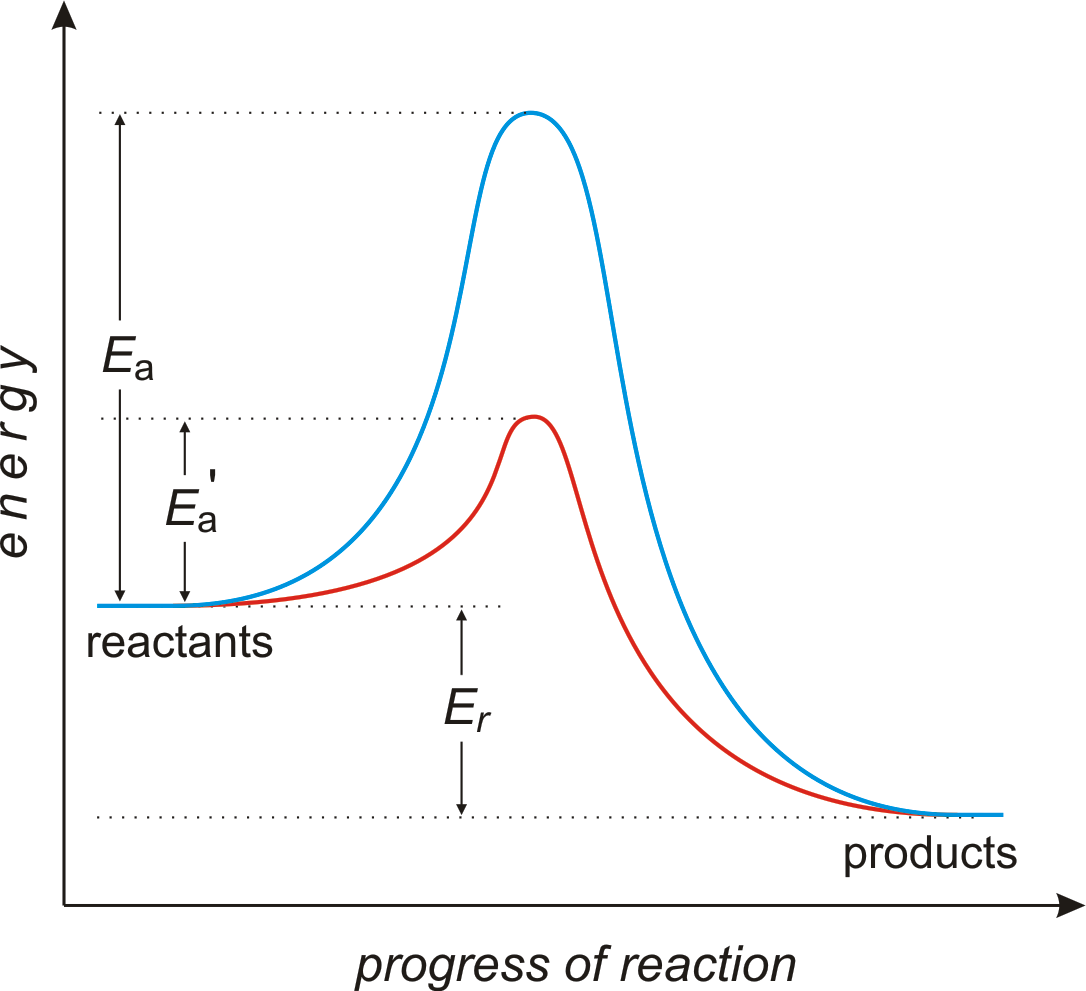
https://finance.yahoo.com/news/ppsi-stock-6-things-know-192319609.html Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated
I can draw, label, and identify potential energy diagrams for both endothermic and exothermic reactions. I can demonstrate the effect of a catalyst on a potential energy diagram. 1. Draw a PE diagram for the synthesis of aluminum oxide (HINT: where can you go to both find this reaction AND determine if its endothermic or exothermic? If you don't

Potential energy diagram with catalyst
A catalyst, or enzyme, works with a substrate to decrease the amount of initial energy required to perform a specific chemical opperation, speeding the reaction up. Enzymes also work to increase ... First time posting and doing some stock research. I came across BKKT with 0 shares available to short according to finitel. Noticed it has high cost to borrow, is on the SSR list and high SI and FTDs. Catalyst, check their twitter. Just announced they’re partnering with bringmethat.com Whatcha all think? Click here to get an answer to your question ✍️ Draw a graph of potential energy v/s reaction coordinate showing the effect of a catalyst on activation ...1 answer · Top answer: The graph of potential energy v/s reaction coordinate showing the effect of a catalyst on activation energy is as shown. A catalyst provides an alternate ...
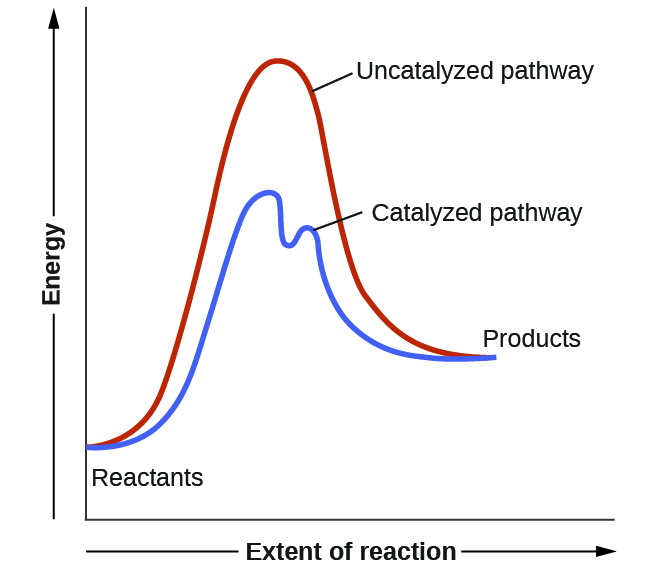
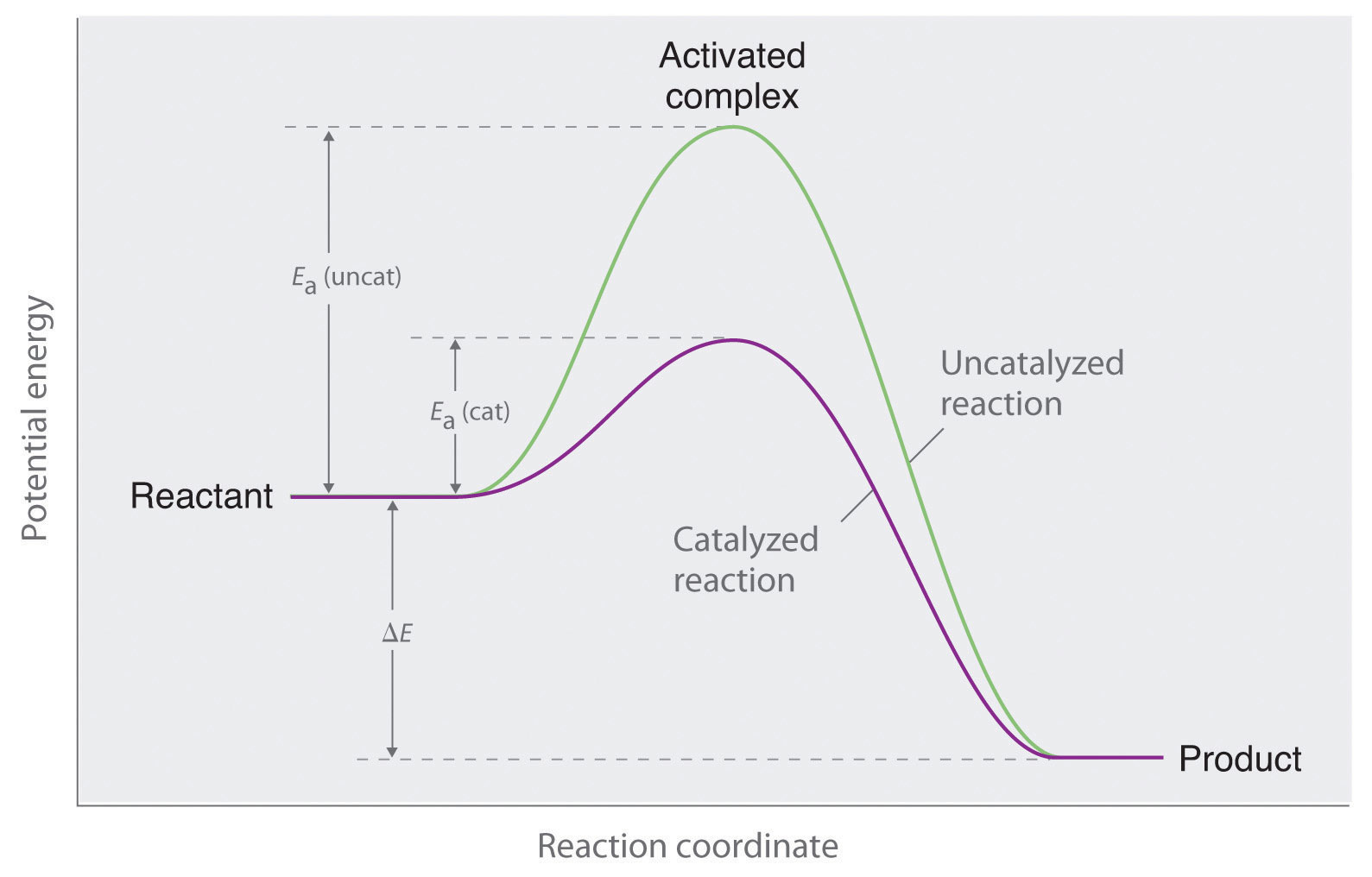
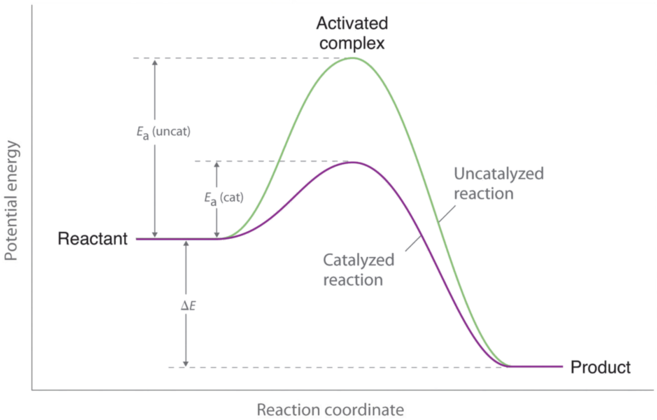
Potential energy diagram with catalyst. The potential energy diagram of a chemical reaction is shown. Interval B represents the A. potential energy of the products C. activation energy B. potential energy of the reactants D. activated complex 30. The combustion of propane is best described as an A. endothermic chemical change C. exothermic chemical change Sep 4, 2021 ... Figure 12.7.2: This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction ... Citius Pharmaceuticals (CTXR) Completes Enrollment in the Pivotal Phase 3 Study of its Cancer Immunotherapy I/ONTAK for the Treatment of Cutaneous T-Cell Lymphoma Good day everyone, **Citius Pharmaceuticals, Inc. (NASDAQ: CTXR)** is a late-stage biopharmaceutical company with a focus on oncology, anti-infectives in adjunct cancer care, unique prescription products, and stem cell therapies. Current price $1.60/share (+6.5% at 10:00 a.m. EST 12-6-21) After closing last Friday’s session at $... This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
Potential Energy Diagram The graph shows a reaction rate with and without the use of a catalyst. Summary. A catalyst is a substance that increases the rate of a chemical reaction. A catalyst provides an alternate pathway for the reaction that has a lower activation energy. When activation energy is lower, more reactant particles have enough ... The potential energy diagram below shows the reaction X + Y → Z. When a catalyst is added to the reaction, it will change the value of 1) 1 and 2 3) 2 and 3 2) 1 and 3 4) 3 and 4 17. According to Table I, the least amount of energy would be evolved by the formation of one mole of A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. Sketch out potential energy diagram with two reaction pathways: one for a reaction without a catalyst and one for the same reaction with a catalyst. FREE Expert Solution 86% (479 ratings)
Catalyst – substance that speeds up a chemical reaction without being consumed. Ex. Enzymes are a biological catalyst speeding up biochemical processes. Page 7 ... Took this from another page but you know the drill from my last few posts on reason for no link back. I'm sure you should find it in a search. Either way, here's the TLDR version and the "meat and potatoes" on what to watch this month: **$TNXP** This month Tonix gives an oral presentation at the American College of Rheumatology Convergence. The event is held between November 5-9 where Tonix presents on its TNX-102SL. While TNX-102 is being studied in COVID, this presentation will focus on TNX-... Arizona Metals is still a table pounding buy with potential to hit $40+ if not acquired well before that point... On January 3rd 2022, exceptional fund manager Michael Gentile was interviewed to discuss some of his mining holdings **(link to interview below)**. I would also read a reddit DD with a grain of salt, so for those who want to hear it from a professional this interview helps simplify the story and portray how much upside there really is. The discussion of AMC starts at the **21 minut... Enrolment numbers should be released within the next week or two, and earnings are predicted for 8th Feb, one month today. If CLOV can deliver better than predicted on both of these, we should see 2 much needed bounces over the next month. This would bring back some confidence in our company, and retail investors will flood back in. All we can do in the meantime is hodl with diamond hands and look forward to the future 🙌🏼💎🍀
Answer: The overall diagram will depend on whether the reaction is endothermic (final H is higher then initial H) or exothermic (final H is lower than initial H) BUT: Catalysed reactions lower the activation energy - the hump that needs to be overcome for the reaction to proceed. (Catalysts ofte...
Why does a potential energy diagram showing the effect of a catalyst on activation energy not move left on the reaction pathway scale (compared to uncatalysed reaction) if a catalyst speeds up reac...
Citius Pharmaceuticals (CTXR) Completes Enrollment in the Pivotal Phase 3 Study of its Cancer Immunotherapy I/ONTAK for the Treatment of Cutaneous T-Cell Lymphoma Good day everyone, **Citius Pharmaceuticals, Inc. (NASDAQ: CTXR)** is a late-stage biopharmaceutical company with a focus on oncology, anti-infectives in adjunct cancer care, unique prescription products, and stem cell therapies. Current price $1.60/share (+6.5% at 10:00 a.m. EST 12-6-21) After closing last Friday’s se...
Potential energy diagrams. ... catalyst provides an alternative reaction pathway which involves less energy and so the catalyst lowers the activation energy. The use of a catalyst does not ...
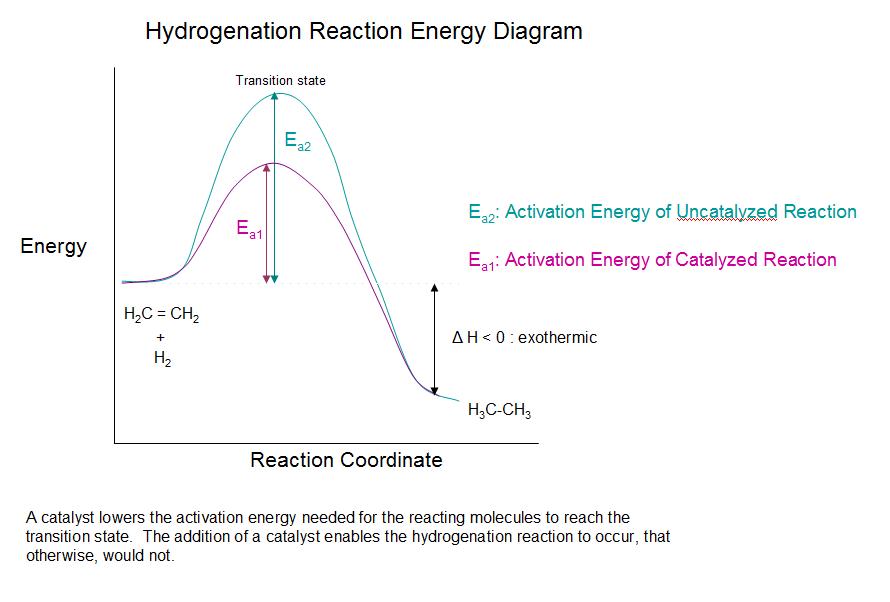
Draw and label a potential energy diagram for a two-step reaction. ... If a catalyst lowered the step one activation energy to a value lower than the step ...
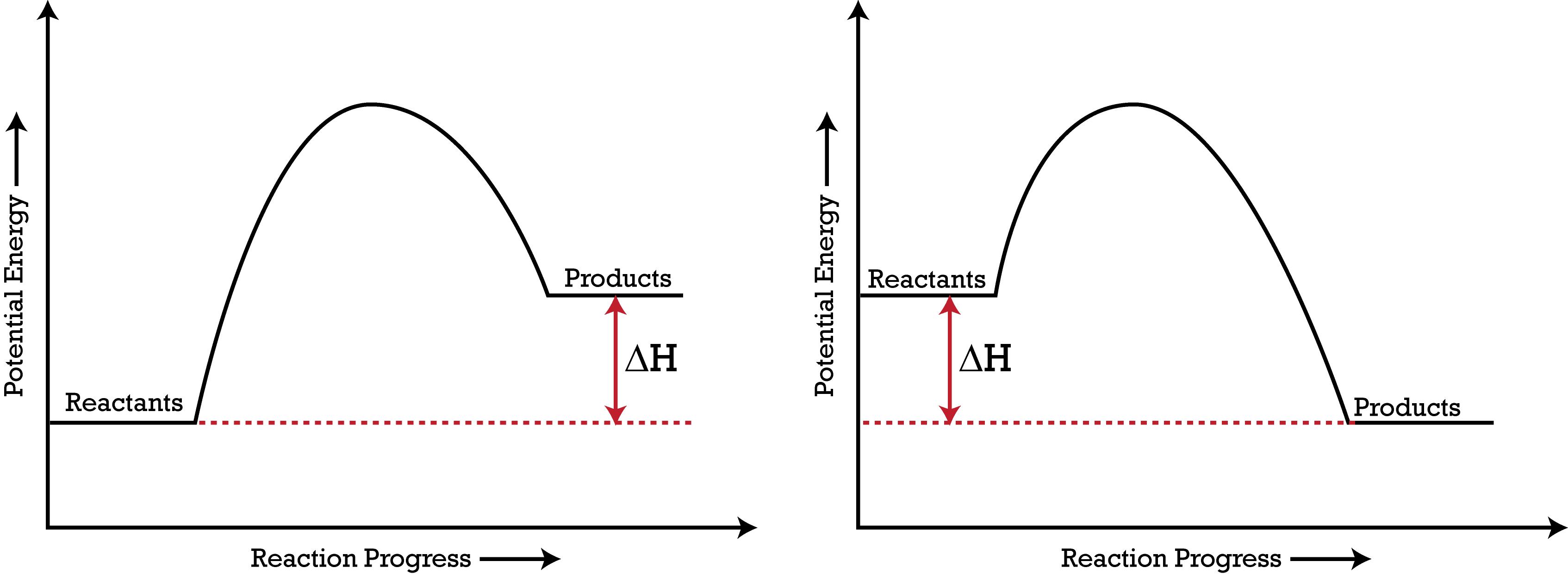
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Potential Energy Diagrams Recall, we have talked about chemical bonds having stored energy (AKA potential energy). For that reason, chemists use diagrams called Potential Energy Diagrams to illustrate the potential (or stored) energy changes that occur during specific chemical reactions. Recall: A reaction is the breaking and reforming of bonds
Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a-f in the diagram represents the potential ... A catalyst changes the reaction mechanism, in the process lowering the activation energy. 5. Name 4 things that will speed up or slow down a chemical reaction.
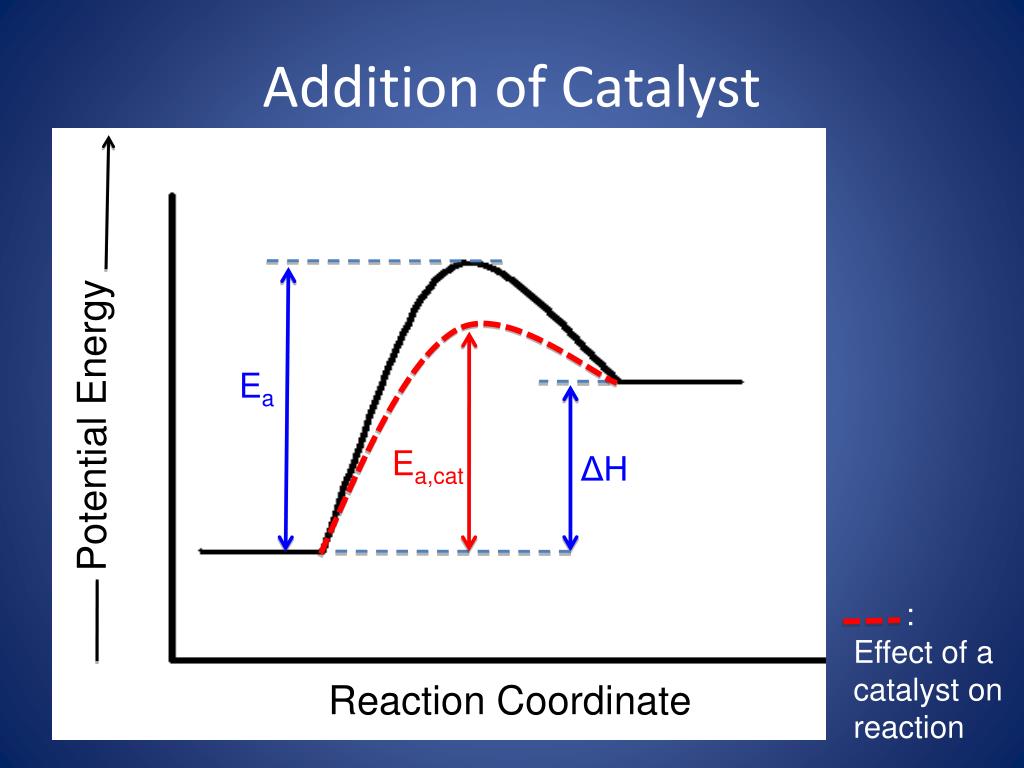
Potential energy barriers for catalyzed and uncatalyzed reactions; The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a.
This graph compares potential energy diagrams for a single-step reaction in the presence and absence of a catalyst. The only effect of the catalyst is to lower the activation energy of the reaction. The catalyst does not affect the energy of the reactants or products (and thus does not affect ΔE).
Potential energy diagram with/without catalyst in a hypothetical exothermic chemical reaction coordinate of Boltzmann distribution. The presence of the catalyst opens a different reaction pathway ...
Stance change would be like Childe, or I suppose even Raiden to a lesser degree with the burst. What do I mean by cheating with catalyst? You could make a catalyst user who is a brawler that fights with their fists. The catalyst could just float behind them, and empower their strikes with their respective element. A firearm Catalyst user, where the Catalyst is used on the firearm like a clip/magazine/etc. (I'm imagining something kind of like how Pyro Fatui Gunners rifles look.) If you change ...
Note: This is my first ever DD on this sub, if you have any tips to improve my future DD's please tell me! Also, this is by no mean financial advise, do your own research. 88 Energy Ltd might be going up alot in the coming few weeks/monhts. Here are some reasons why: The company has had a negative net profit for the last couple of years, with a pretty bad loss of AUD36 million in 2019. Since then the stock price dropped pretty hard. They haven't sold anything in the last couple of years and th...
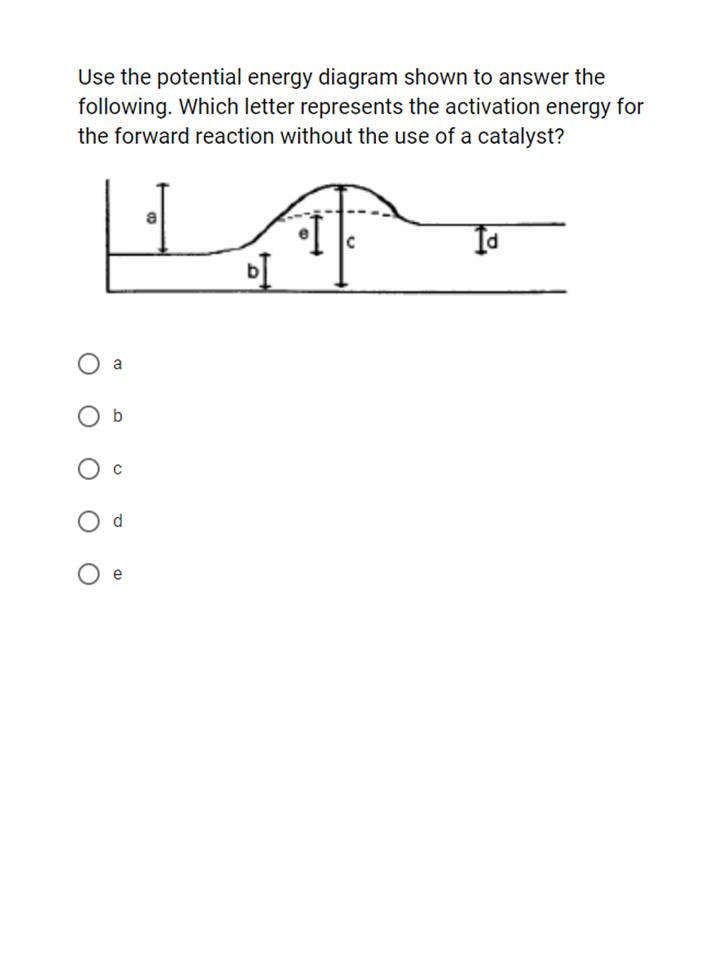
Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain whv.] According to the diagram, is the potential eneoy of the products greater than, less than, or equal to the potential energy of the reactants? Draw an alTOW on the diagram above to represent the activation energy for the forward reaction. Label the
Energy diagrams are useful to illustrate the effect of a catalyst on reaction rates. Catalysts decrease the activation energy required for a reaction to proceed ...
Catalyst - the activation energy is lowered for the reaction, ... The graph below represents the relationship between potential energy and course of reaction for a ... C. 3 kJ D. 4 kJ Question 3 A certain chemical reaction is represented by the potential energy diagram below. Which ONE of the following quantities will change when a catalyst ...
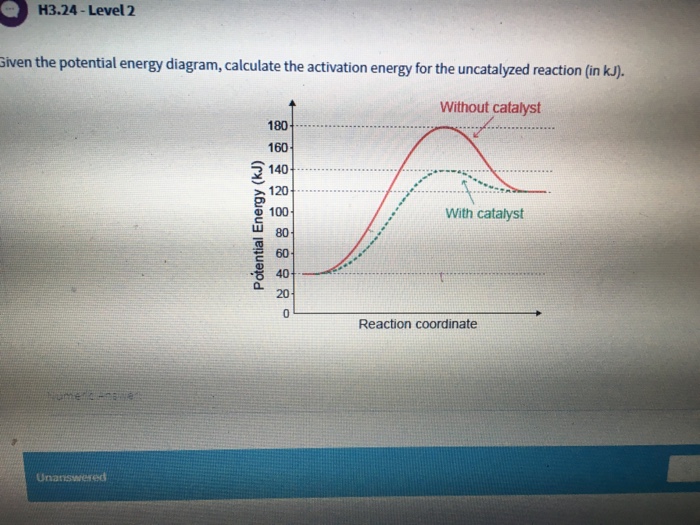
S(s) + 02(g) S02(g) + energy Which diagram best represents the potential energy changes for this reaction? 12. Given the potential energy diagram for a reversib chemical reaction: Reaction Coordinate Each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kilojoules per mole. Wha is the activation energy of the forward ...
1. In the potential energy diagram, which letter represents the potential energy of the activated complex? A. A B. B C. C D. D 2. The addition of a catalyst to a reaction will cause a change in the A. potential energy of the reactants B. potential energy of the products C. heat of reaction D. activation energy 3.
7. What letter represents the potential energy of the activated complex? 8. Is the reverse reaction endo or exothermic? 9. If a catalyst were added, what letter(s) would change? a e c a and c 12. POTENTIAL ENERGY DIAGRAM WS 1. Which of the letters a-f in the diagram represents the potential energy of the products? _____ 2.
EEENF 88 energy limited has dropped down in value to around .01 and has a good possibility of going to at least 0.04 by June. This is a Australian oil company and has found a large amount of oil in Alaska and will do its spud on March 8th. https://88energy.com/ https://finance.yahoo.com/quote/88E.AX/
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
Progenity, Inc. engages in the provision of molecular and specialized diagnostic tests to clinicians. Its products include Preparent Carrier Test, Innatal Prenatal Screen, Riscover Hereditary Cancer Test, and Resura Prenatal Test. Case for Short-Squeeze 1. Prog has one of the highest SI (about 64%) while only one entity is trying to keep the price suppressed (Athyrium). 2. CTB continues to rise everyday as per Ortex data 3. Ortex has issued all 3 signals of short-squeeze on Prog 4. It’...
A regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. The dotted line shows a new potential energy diagram with two activation energy hills, the second taller than the first, instead of the one hill in the regular potential energy diagram. A ...
potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed reaction has to overcome a substantial energy barrier, whereas the barriers in the catalytic route are much lower. The energy diagram of Fig. 1.2 illustrates ...
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction.
Sketch the potential energy diagram for an exothermic reaction with a catalyst added.
Click here to get an answer to your question ✍️ Draw a graph of potential energy v/s reaction coordinate showing the effect of a catalyst on activation ...1 answer · Top answer: The graph of potential energy v/s reaction coordinate showing the effect of a catalyst on activation energy is as shown. A catalyst provides an alternate ...
First time posting and doing some stock research. I came across BKKT with 0 shares available to short according to finitel. Noticed it has high cost to borrow, is on the SSR list and high SI and FTDs. Catalyst, check their twitter. Just announced they’re partnering with bringmethat.com Whatcha all think?
A catalyst, or enzyme, works with a substrate to decrease the amount of initial energy required to perform a specific chemical opperation, speeding the reaction up. Enzymes also work to increase ...

















0 Response to "37 potential energy diagram with catalyst"
Post a Comment