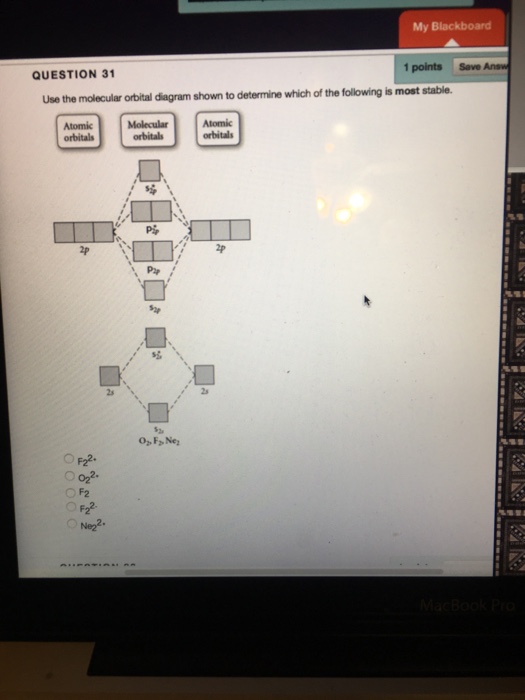
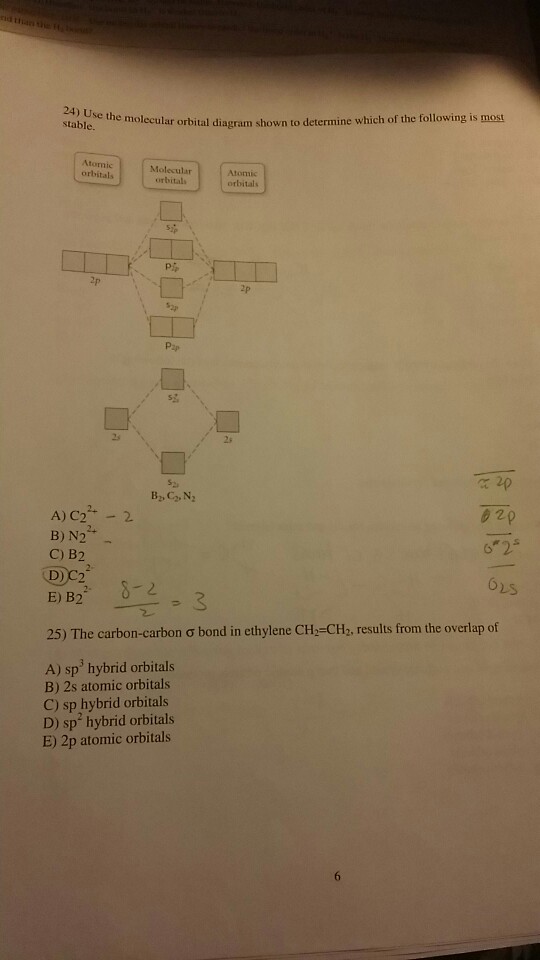
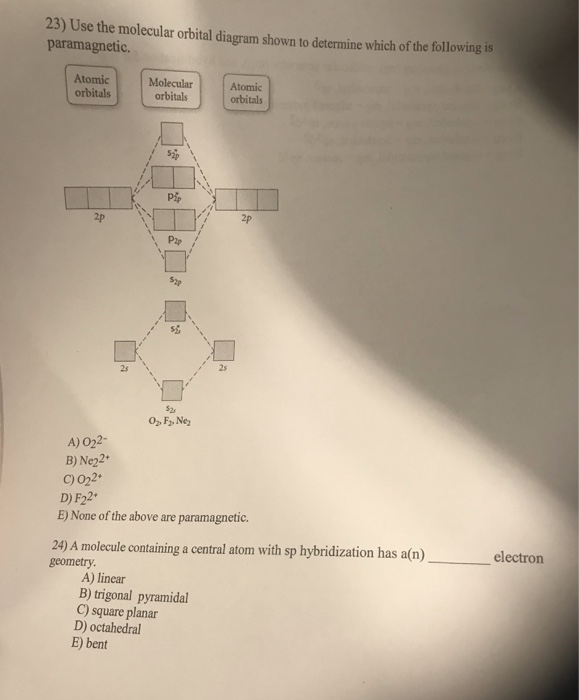
37 use the molecular orbital diagram shown to determine which of the following is most stable
2 hours ago Problem: Use the molecular orbital diagram shown to determine the bond order for NO +. 3 draw the molecular orbital diagram needed and determine which of the following is Preview / Show more. Category: Free Catalogs Show details. Solved Part A Use the molecular... ## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance:This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as protons and neut...
Based on this, which of the following conclusions seems most plausible? Feature scaling speeds up gradient descent by avoiding many extra iterations that are required when one or more features take on much larger values than the rest.

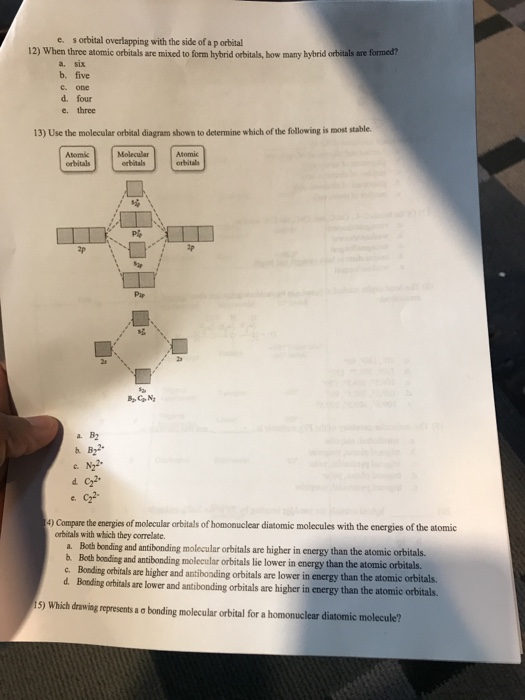
Use the molecular orbital diagram shown to determine which of the following is most stable
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons … Show transcribed image text show the orbital filling diagram for n nitrogen. 3. The Molecular Orbital Theory (MOT) is a theory on chemical bonding developed at the beginning of the twentieth century by F. Draw a correct Lewis structure for boric acid, B(OH)3. For oxygen and fluorine, the σ2 p orbital should be lower in energy than the π2 p ... Many scouting web questions are common questions that are typically seen in the classroom, for homework or on quizzes and tests. Some questions will include multiple choice options to show you the options involved and other questions will just have the questions and corrects answers.
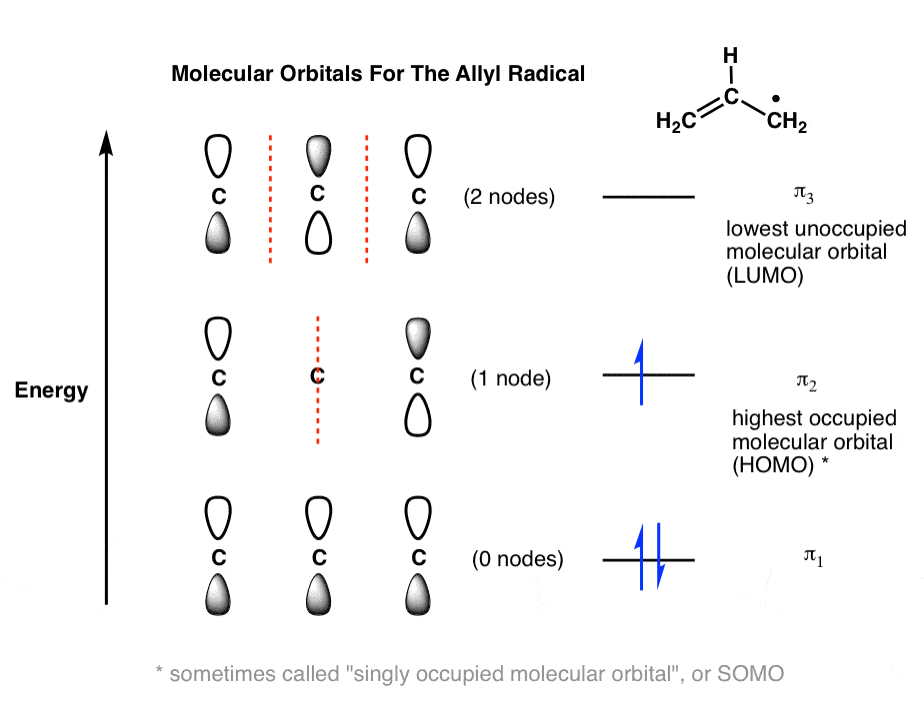
Use the molecular orbital diagram shown to determine which of the following is most stable. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to Molecular Orbital Theory: Tutorial and Diagrams - Video. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. ion is most stable than [Math Processing Error]. H2+,H+,H. . Because s-orbital is fulfilled of [Math Processing Error]. Fill in the molecular orbitals in the molecular orbital diagram for CO. One 2 s and three 2 p orbitals from carbon and one 2 s and three 2 p orbitals combine to form eight molecular orbitals in C O. The molecular orbitals in order of increasing energy are one sigma 2 s, one sigma 2 s star, two pi 2 p, one sigma 2 p, two pi 2 p star, and one ... Feb 16, 2017 · The “lowest unoccupied” molecular orbital is π 2 . We generally abbreviate the terms “highest occupied molecular orbital” as HOMO and “lowest-unoccupied molecular orbital ” as LUMO. They are often called the “frontier” molecular orbitals and are where most of the action happens in reactions, as we’ll see in future posts. 7.
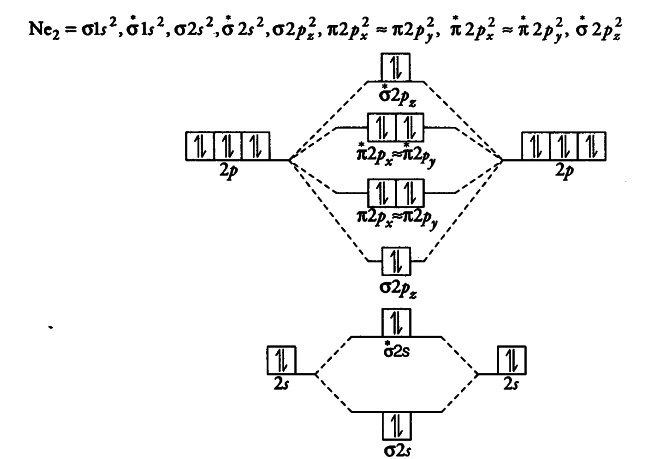
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. Using the molecular view of the tlc plate provided. Label each and each and every molecular orbital with its call sigma pi and position the accessible electrons interior the perfect atomic orbitals and 1 draw the molecular orbital diagrams to determine which of the following is most stable. Use a qualitative molecular orbital energy-level diagram to predict the valence electron configuration, bond order, and likely existence of the Na 2 − ion.365 MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions. Molecular orbital may, therefore, be defined as "the region in space associated with all the nuclei of the molecule where the probability of finding a particular electron is maximum". As in the case of atomic orbitals, each molecular orbital can accommodate at the most two electrons with opposite...
Who are the experts?Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. jmbeachthub 1 draw the molecular orbital diagrams to determine which of the following is most stable a f2 b f2 2 c ne2 2 d o2 2 For this problem refer a n 2 2 b b 2 c b 2 2 d c 2 2 e c 2 2 Download high res image chapter 10tif chemical polarity to determine which of the following is most stable... How many arguments does the following method accept? Which of the following characters is the wild card character that is used to import all the classes in a particular package? A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.. In the kinetic theory of gases, the term molecule is …
Science Chemistry Q&A Library Which of the following is correct for reversible or irreversible compression of an ideal gas (from V1 to V2): (A) reversible work = irreversible work (B) reversible work < irreversible work (C) reversible work > irreversible work (D) w=negative sign (E) reversible work...
Use the molecular orbital diagram shown to determine which of the following is most stable. Which of the following resonance structures for OCN- will contribute most to the correct structure of OCN? Using molecular orbital theory, determine whether F2, F2−, or (F2)^2− would have the...
Need vacuum diagram for 2002 ranger 23l ford ranger forum. 2000 2.5 - Ranger-Forums - The Ultimat... Through the thousands of pictures on line with regards to 2001 ford explorer sport fuse box diagram picks the top libraries using best r... Follow the sign convention.
90. Which of the following are commonly used network topologies? 91. When two devices communicate, they must agree on protocols for I.starting and ending a transmission II.recognizing transmission errors III.determining the rate of data transmission.
Using molecular orbital theory, explain why the removal of one electron in O2 strengthens bonding, while The following the diagrams of O2 and F2 respectively: Go through the rules of MOT . To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in...
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures.
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison Use the molecular orbital diagram shown to determine which of the following is most stable.
1 draw the molecular orbital diagrams to determine which of the following is most stable. Determine the magnetism of simple molecules. Label each and each and every molecular orbital with its call sigma pi and position the accessible electrons interior the perfect atomic orbitals and...
Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
The maximum number in which no more than 8 bits of information are included. Варианты ответов Варианты ответов: *device for direct perception of the information to the person. *information containing on a hard disk.
You can use the periodic table as reference. El … ement Electron Orbital Filing Diagram Highest Number Bored talaga laro mona ako ng GTA HAHA. Which of the following pairs of atoms is most likely to This site is using cookies under cookie policy . You can specify conditions of storing and...
The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding.
​ ## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance: This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as...
Sep 04, 2021 · Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, …
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule.
The product of a Lewis acid-base reaction, is a neutral, dipolar or charged complex, which may be a stable covalent molecule. As shown at the top of the following drawing, coordinate covalent bonding of a phosphorous Lewis base to a boron Lewis acid creates a complex in which the formal charge of boron is negative and that of phosphorous is ...
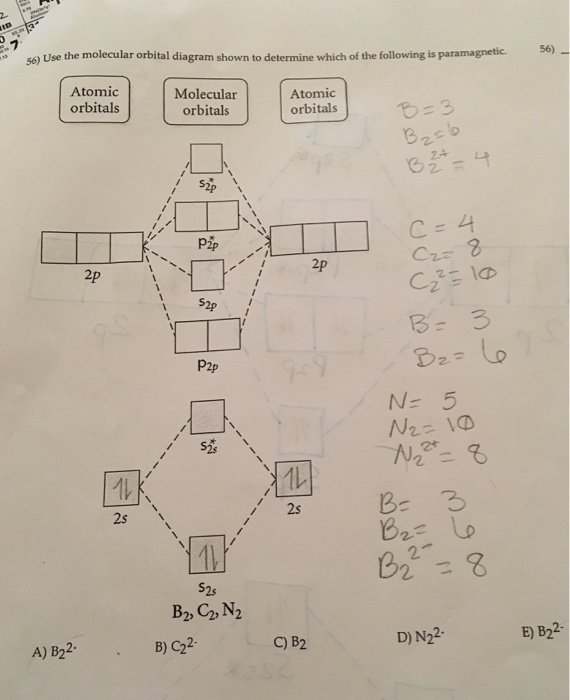
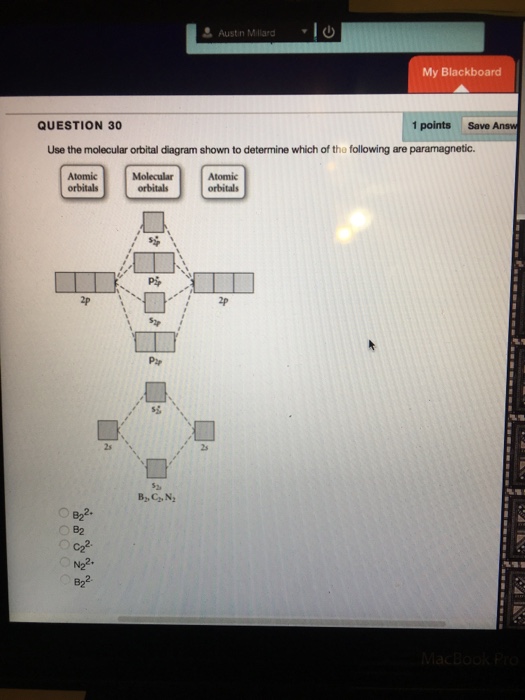
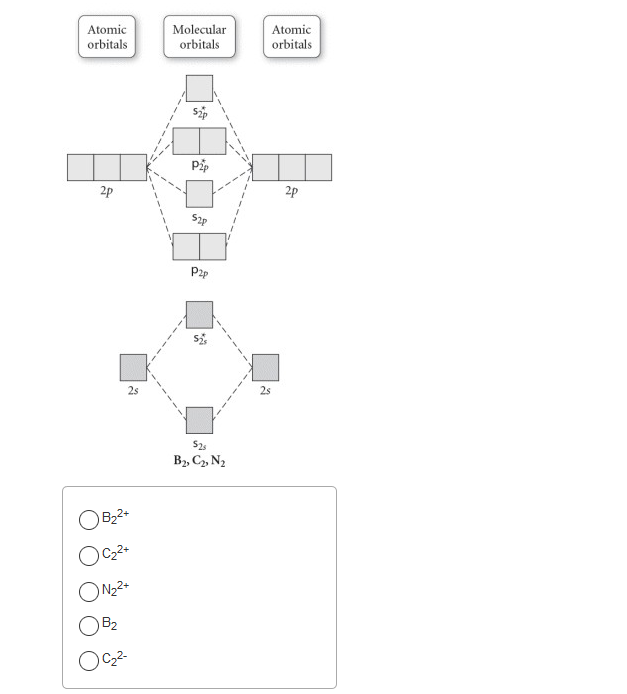
15 55) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. 16 58) Determine the molecular geometry about each interior atom in the following structure. Sketch the 3 dimensional structure and label the interior atoms with the corresponding...
A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. Talking about the overlap diagram of H3O+, it is almost similar to H2O but Learning the basics will help you to understand the reactions more and have a clear picture of the hydronium ion.
Use the molecular orbital diagram shown to determine which of the following is most stable. A n 2 2. Introduction To Molecu...
Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule.
Answer: C 11 52) Use the molecular orbital diagram shown to determine which of the following is most stable. If the molecular geometry causes all of the dipoles to cancel, the molecule will be nonpolar. An example is CF4 where there are four polar bonds, but the dipoles sum to 0 making the...
Many scouting web questions are common questions that are typically seen in the classroom, for homework or on quizzes and tests. Some questions will include multiple choice options to show you the options involved and other questions will just have the questions and corrects answers.
Show transcribed image text show the orbital filling diagram for n nitrogen. 3. The Molecular Orbital Theory (MOT) is a theory on chemical bonding developed at the beginning of the twentieth century by F. Draw a correct Lewis structure for boric acid, B(OH)3. For oxygen and fluorine, the σ2 p orbital should be lower in energy than the π2 p ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons …

















0 Response to "37 use the molecular orbital diagram shown to determine which of the following is most stable"
Post a Comment