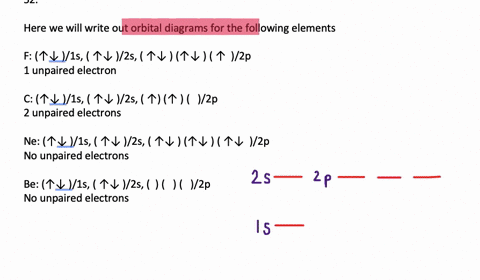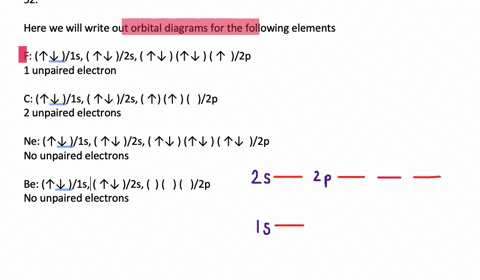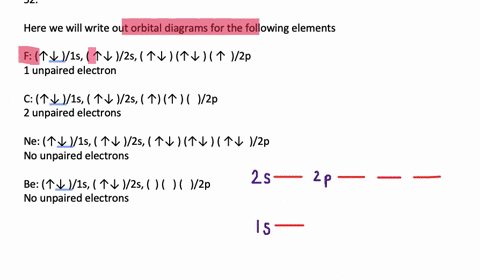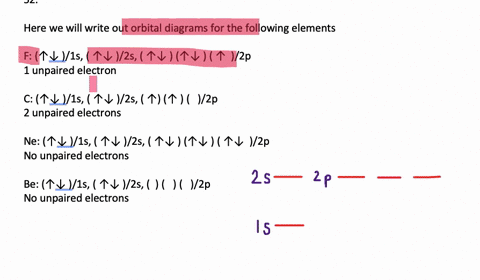
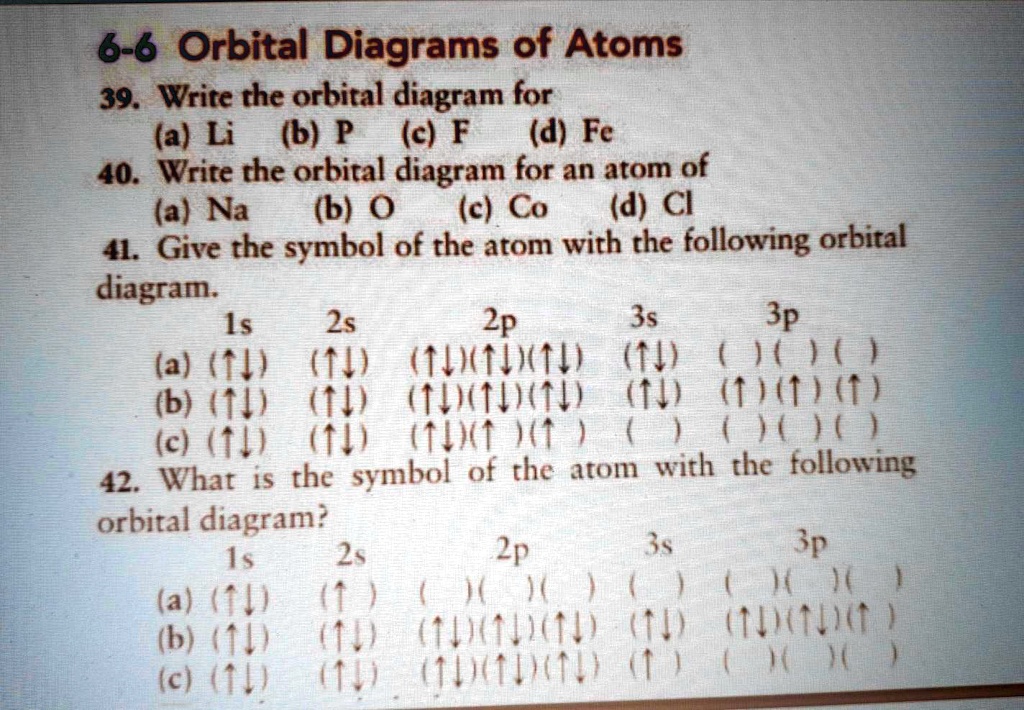
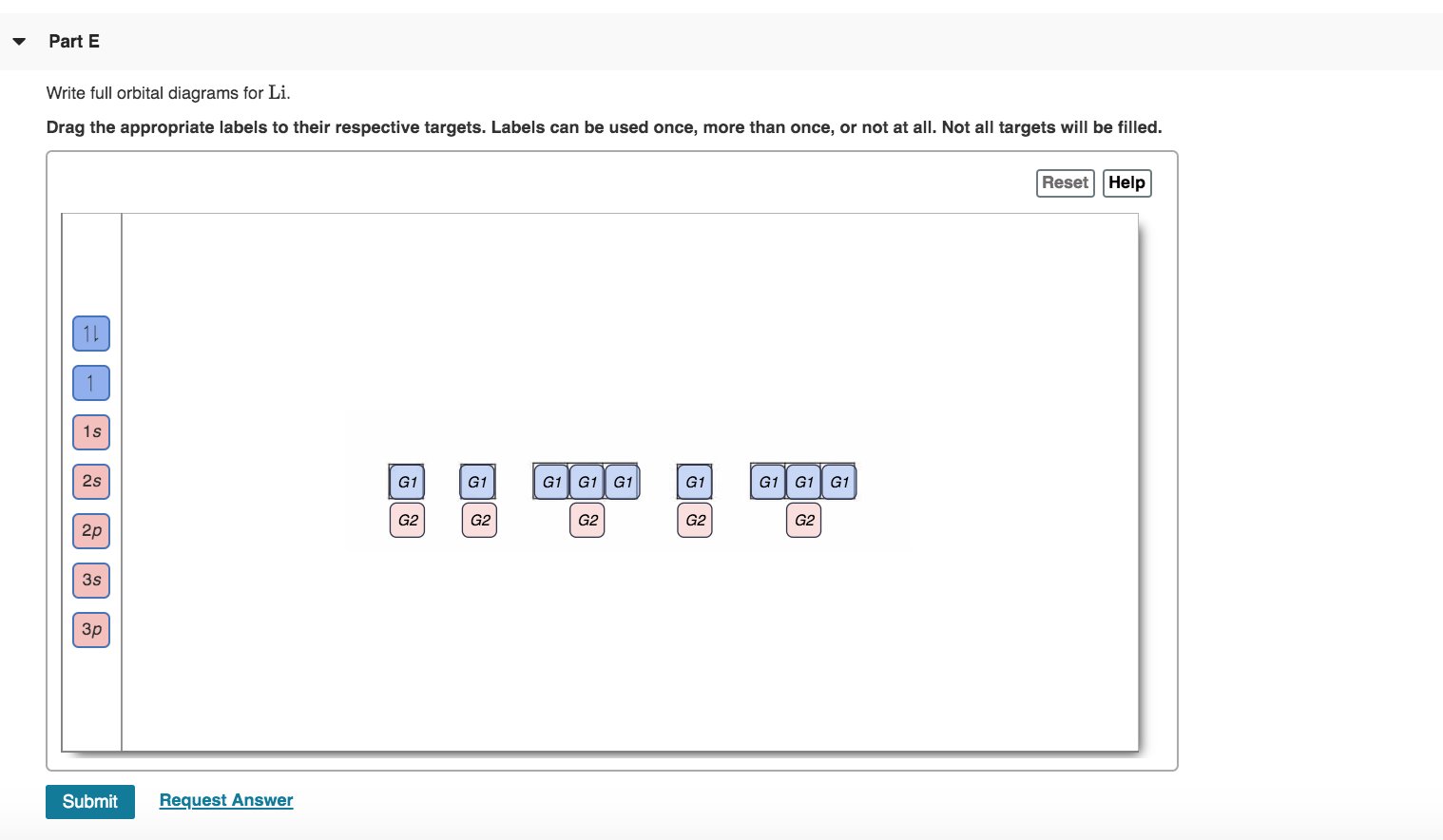
40 write full orbital diagram for f.
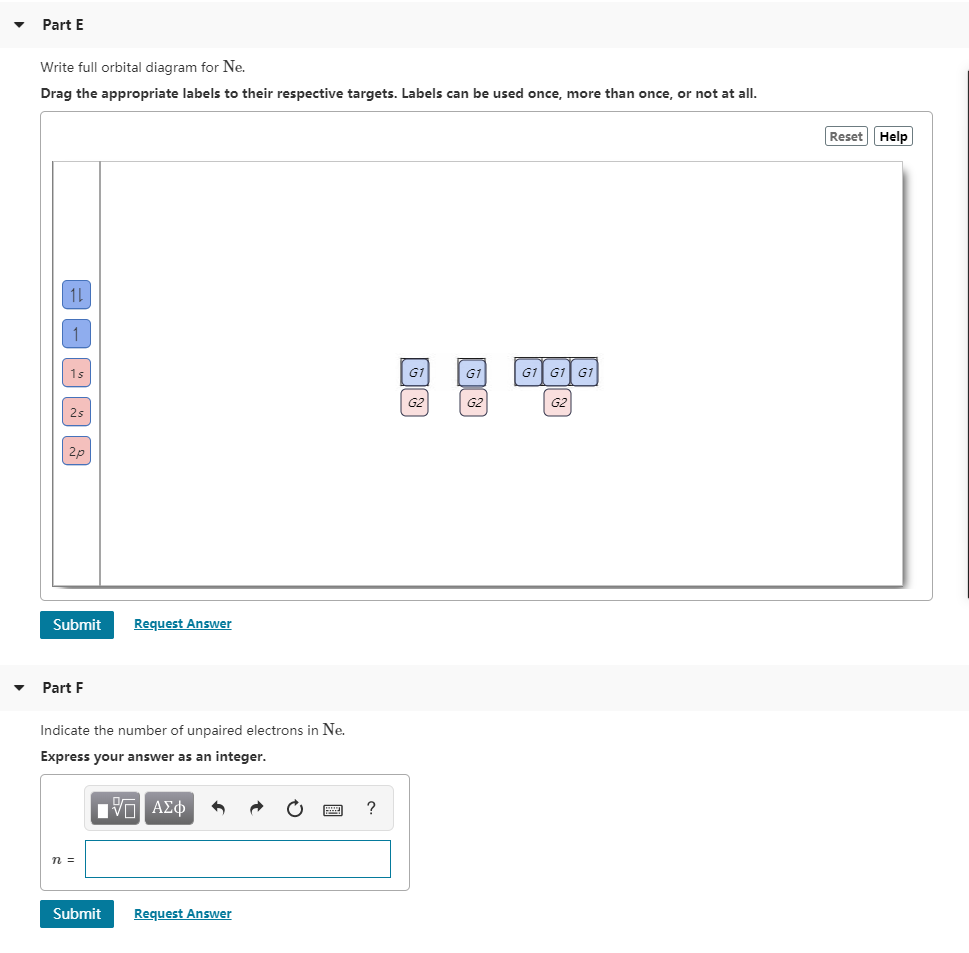
Transcribed image text: Part A Write the full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help 11 1s G1 G1 G1 G1 G1 G2 G2 G2 2s 2p Write the full orbital diagram for C. Drag the appropriate labels to their respective targets. Q. Write orbital diagrams for each of these ions.V5+ Q. Enter the orbital diagram for the ion Zr2 +. Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2.
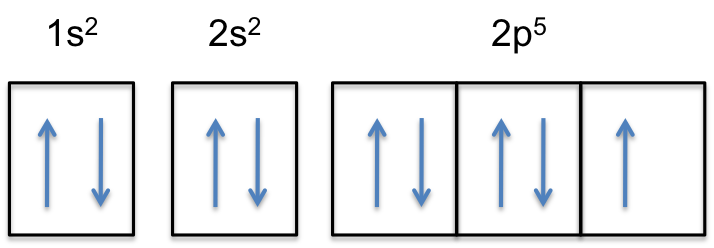
Transcribed image text: Write the full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. TL 1s 11 11 11 1 1 1s 2s 25 2p 2p Part B Indicate the number of unpaired electrons in F. Express your answer as an integer.

Write full orbital diagram for f.
An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up) How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. Correct Part F Indicate the number of unpaired electrons in it. Express your answer as an integer. ANSWER: ANSWER: = 0 Correct Part G Write full orbital diagram for. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
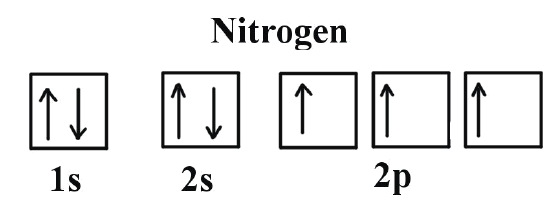
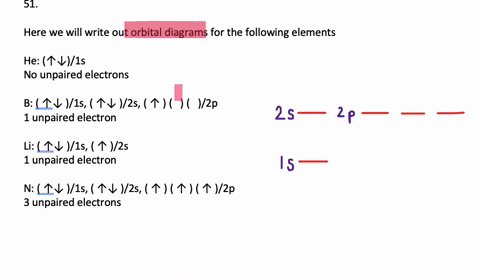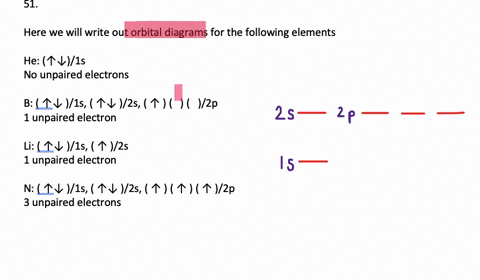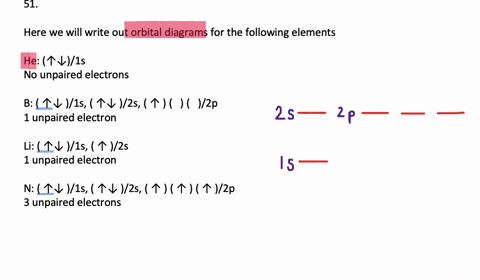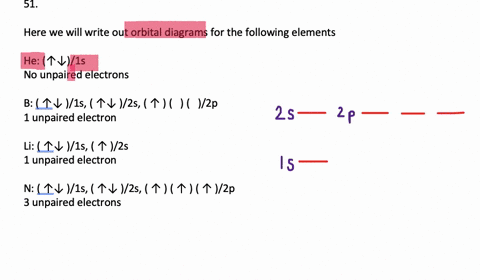
Write full orbital diagram for f.. To write the orbital diagram for the Fluorine atom (F) first we need to write the electron configuration for just F. To do that we need to find the number o... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Write full orbital diagrams and indicate the number of unpaired electrons for: N Write electron configuration and use the symbol of the previous noble gas in brackets to represent core electrons: An oxygen atom has a total of eight electrons. Write the full electron. configuration and the orbital diagram, and the four quantum numbers for each of the eight electrons in the ground state.
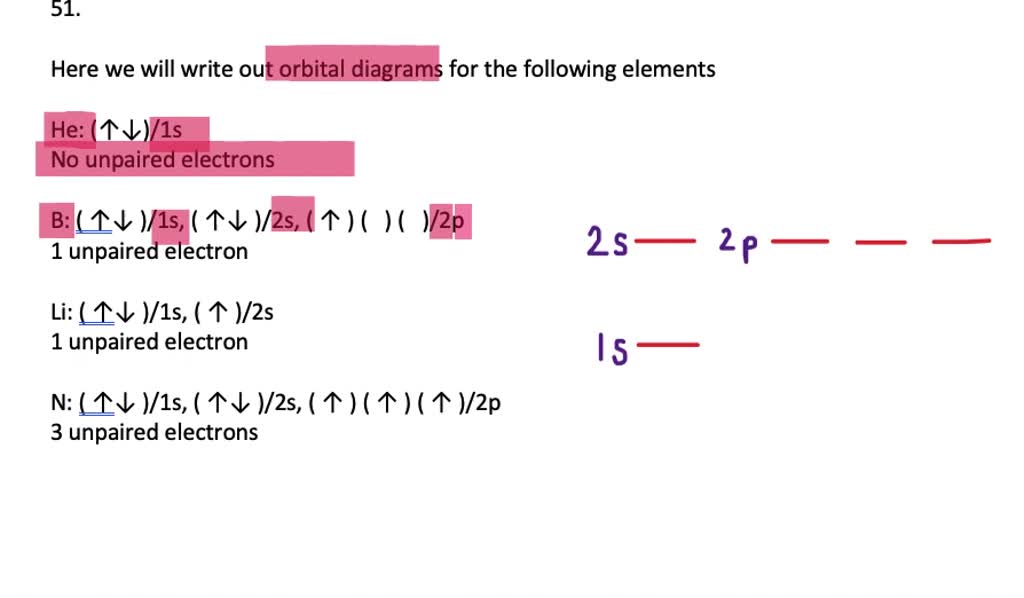
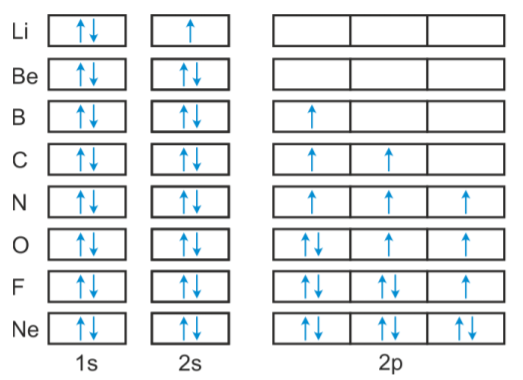
VIDEO ANSWER: Chapter three Problem. Forty eight since right before. No diagram for each element. So the first one they give us Hey, Sulphur. So we were right in the orbit of diagram. I'm going to write it an order Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Answer to: (a) Write the full orbital diagram for F. (b) Indicate the number of unpaired electrons in it. By signing up, you'll get thousands of... Solution for Write the full orbital diagram for element. F
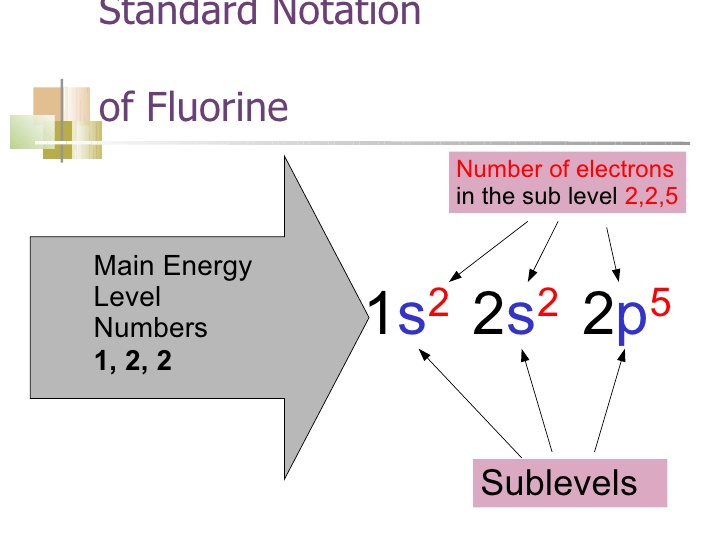
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ... How to Write the Electron Configuration for Boron. Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Correct Part F Indicate the number of unpaired electrons in it. Express your answer as an integer. ANSWER: ANSWER: = 0 Correct Part G Write full orbital diagram for. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital.
An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up)







/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)












0 Response to "40 write full orbital diagram for f."
Post a Comment