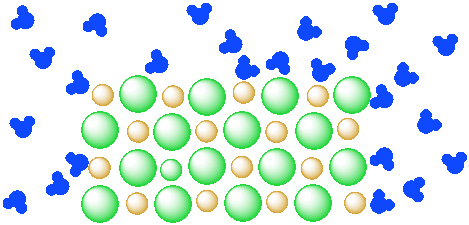
36 diagram of salt dissolved in water
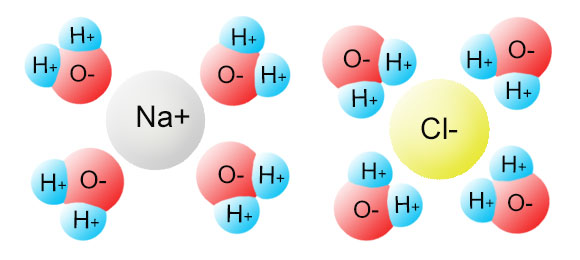
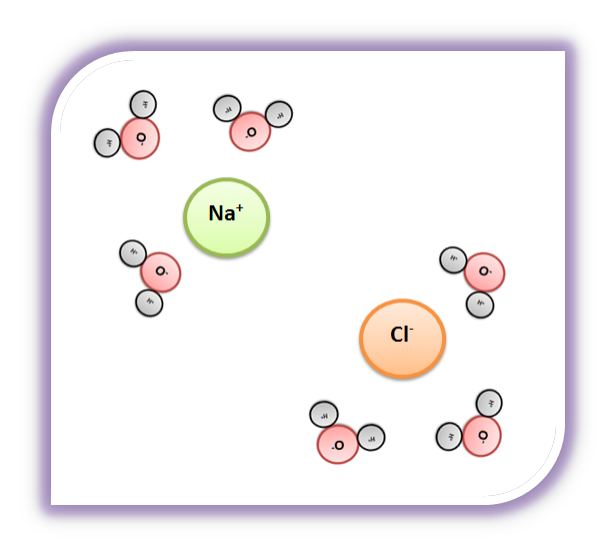
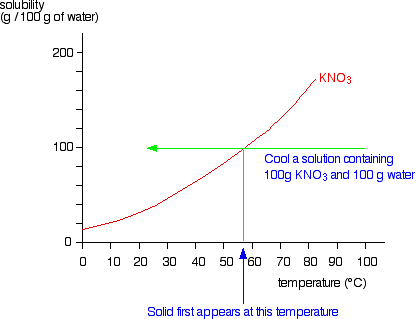
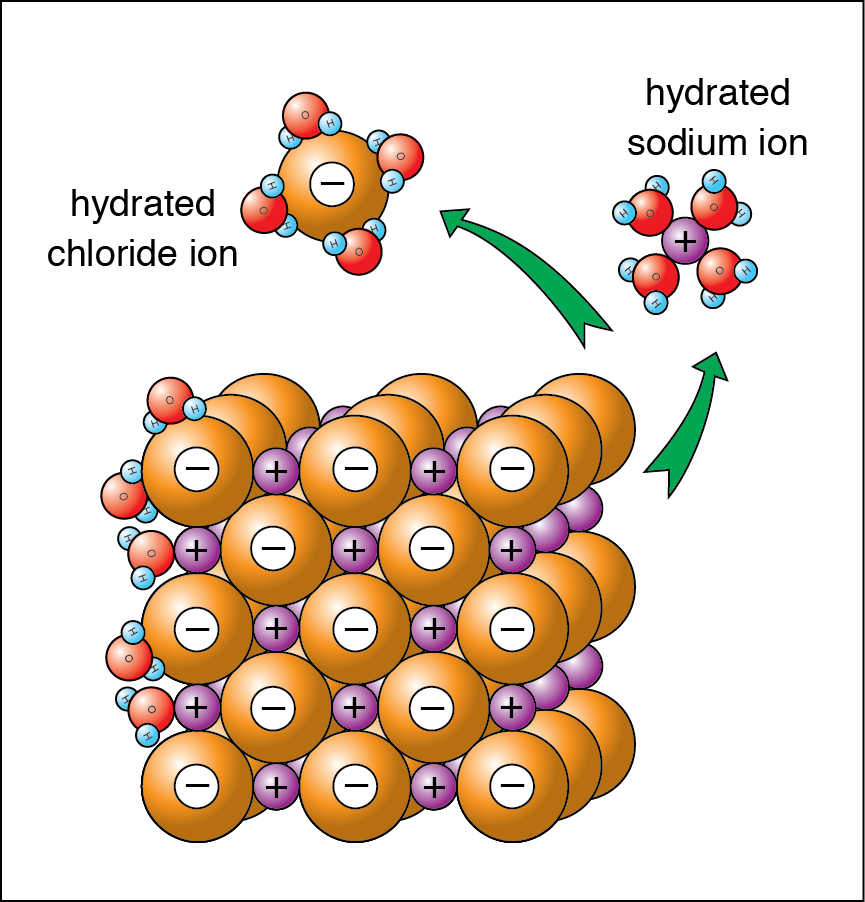
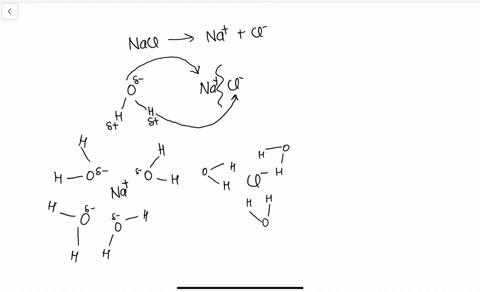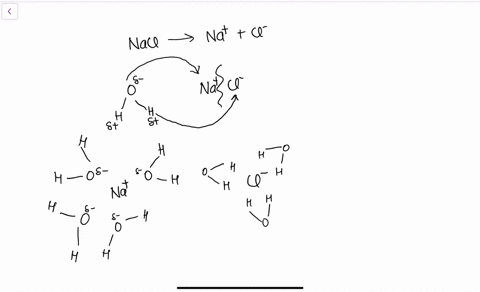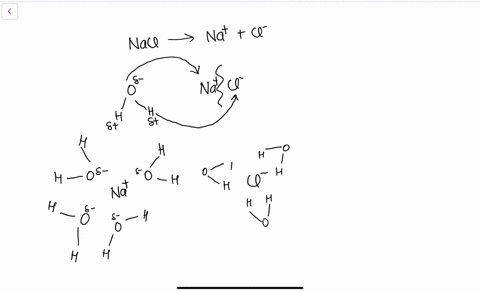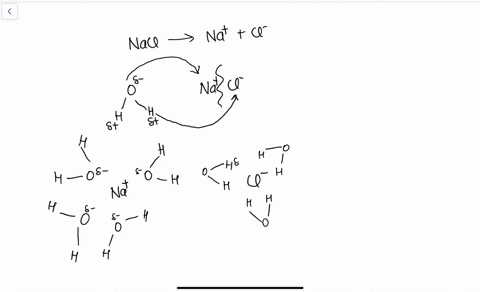
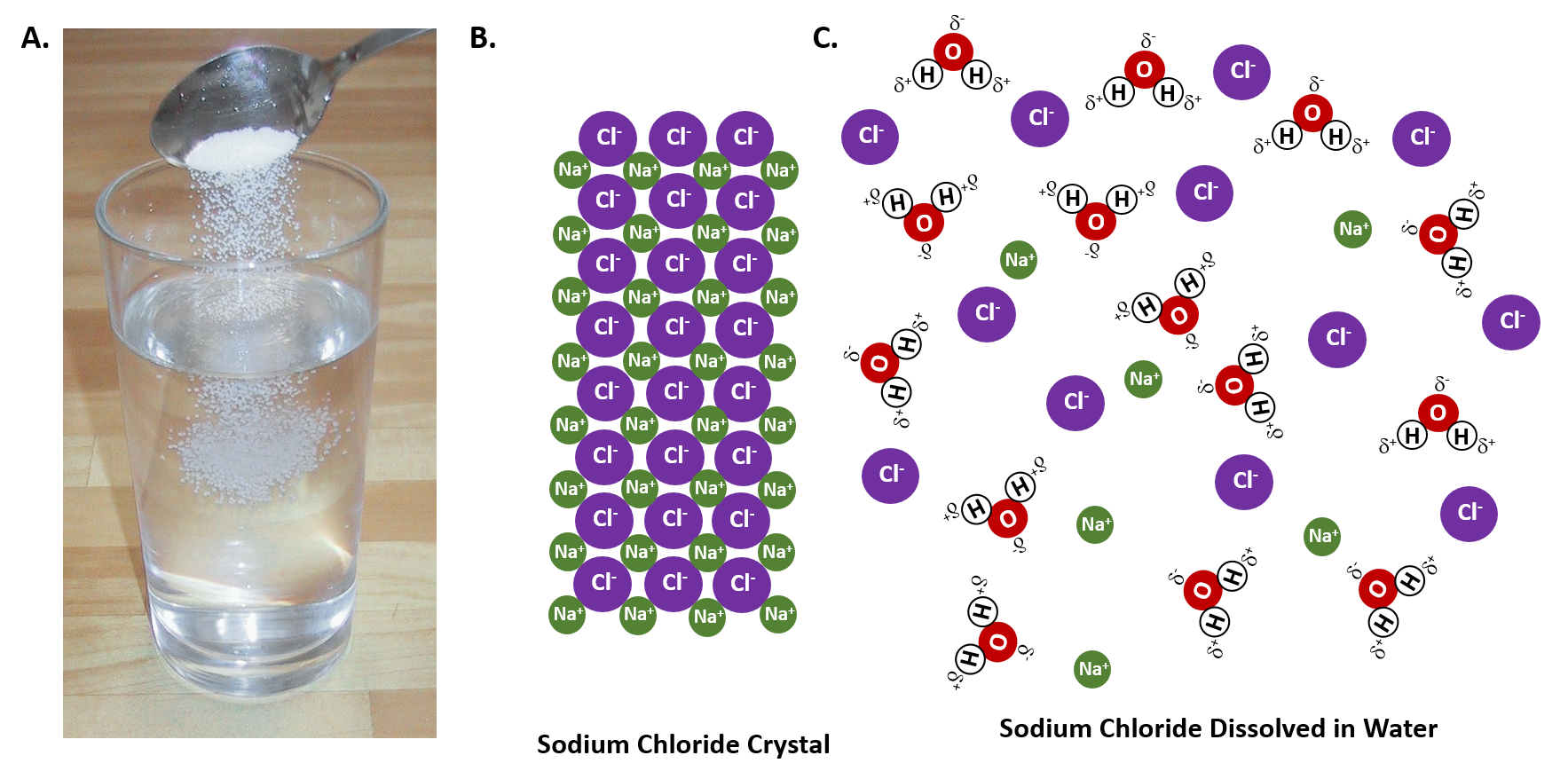
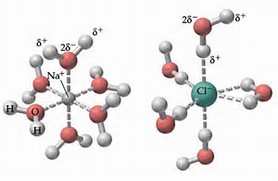
Water, the Universal Solvent | U.S. Geological Survey Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Liquid-Solid Phase Diagrams: Salt Solutions - Chemistry ... Let's take the lines one at a time. The first one to look at is the one in bold green in the next diagram. This line represents the effect of increasing amounts of salt on the freezing point of water. Up to the point where there is 23.3% of salt in the mixture, the more salt the lower the freezing point of the water.
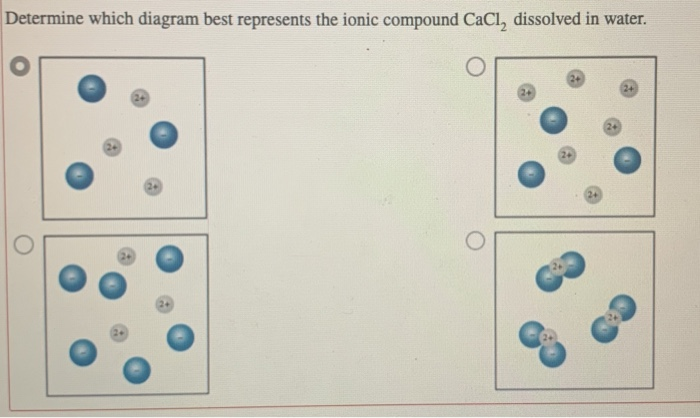
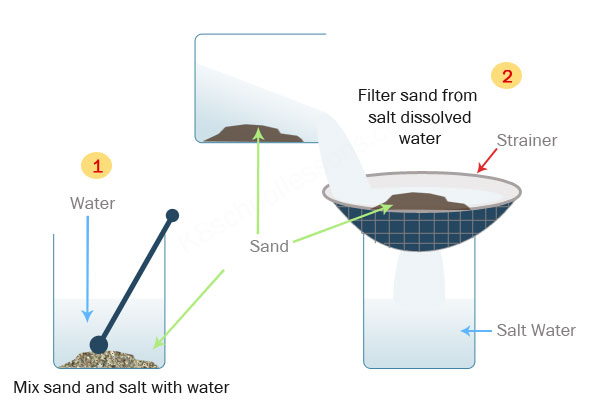
Draw a diagram of table salt (NaCl) dissolved in water ... Find step-by-step Biology solutions and your answer to the following textbook question: Draw a diagram of table salt (NaCl) dissolved in water..
Diagram of salt dissolved in water
What dissolves in salt ionic? - JacAnswers What dissolves in salt ionic? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Solubility Diagram - ScienceGeek.net Solubility Diagram. Show all questions. 1 / 12. At approximately what temperature does the solubility of sodium chloride, NaCl, match the solubility of potassium dichromate, K 2 Cr 2 O 7? 83 ºC. 60 ºC. 50 ºC. 30 ºC. Which salt is LEAST soluble at 0 ºC? Seawater - Wikipedia Seawater, or salt water, is water from a sea or ocean.On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/l, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na +) and chloride (Cl −) ions).Average density at the surface is 1.025 kg/l.
Diagram of salt dissolved in water. Why Does Water Dissolve Salt? | Chapter 5: The Water ... Students will make a 2-D model of a salt crystal and use water molecule cut-outs to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Objective How Does Salt Dissolve in Water? - Reference.com Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds a slight positive or negative electrical charge. These ends attract the positive and negative ions in salt and pull them apart from each other. The polarity of water comes from the differences in electronegativity in ... scioly.org › wiki › indexDynamic Planet/Earth's Fresh Water - Wiki - Scioly.org Feb 22, 2022 · Freshwater is water that has less than 0.2% of dissolved salts. Comparatively, freshwater is relatively scarce, comprising less than 3% of the Earth’s water supply. In addition, the majority of that, about 2%, is frozen away in glaciers and icecaps. Fresh water can also be found in lakes, rivers, streams, atmospheric vapor, and groundwater. What Happens When Salt Is Added to Water? - Sciencing "Salt dissolved in water" is a rough description of Earth's oceans. In chemistry, it results in a solution, as the ionic bond of NaCl is pulled apart by the attraction of Na to the O of H2O and the attraction of Cl to the H of H2O. Very little to no acid is produced in this solution.
FAQ: What kind of change occurs when salt dissolves in water? What happens when salt dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. Video Transcript. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared ... Dissolving Salt in Water: Chemical or Physical Change? Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does not result in a chemical reaction. How Water Dissolves Salt - YouTube Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then dissolving the salt. ... Water molecules pulling apart the ions (sodium and chloride) in a salt crystal ...
Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Dissolving - BBC Bitesize Salt dissolves when it is stirred into water. In sea water, the water is the solvent and the salt is the solute. In sea water, the water is the solvent and salt is the solute. Solid-liquid Phase Diagrams: Salt Solution This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds, such as UK A level chemistry. LS7A Launchpad Week 2 Diagram | Quizlet When salt is dissolved in water, the water dilutes the salt, but the salt also dilutes the water. ... The diagram below shows a cell with three different membrane transport proteins. The Na+/K+ Pump is a primary active transporter and the Na+/Waste Co-transporter is a secondary active transporter. Arrows show the direction of net movement of ...
What happens when salt dissolves in water ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
44 diagram of salt dissolved in water - Wiring Diagram Source Diagram of salt dissolved in water Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
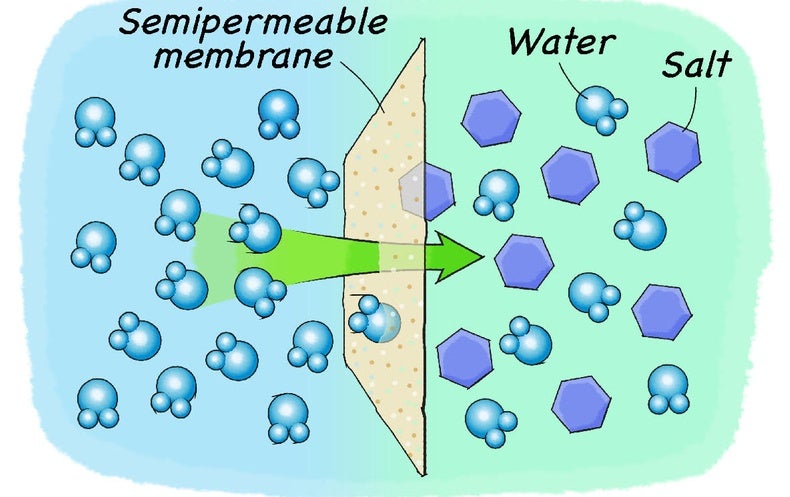
› reverse-osmosis-rejectRO Reject Water Disposal - Reverse Osmosis (RO) Water ... Reverse Osmosis (RO) water treatment technology has been used for years in various industries to separate dissolved solids from water by forcing the water through a semi-permeable membrane. RO reject water disposal is also commonly used to purify drinking water and desalinate seawater to yield potable water.
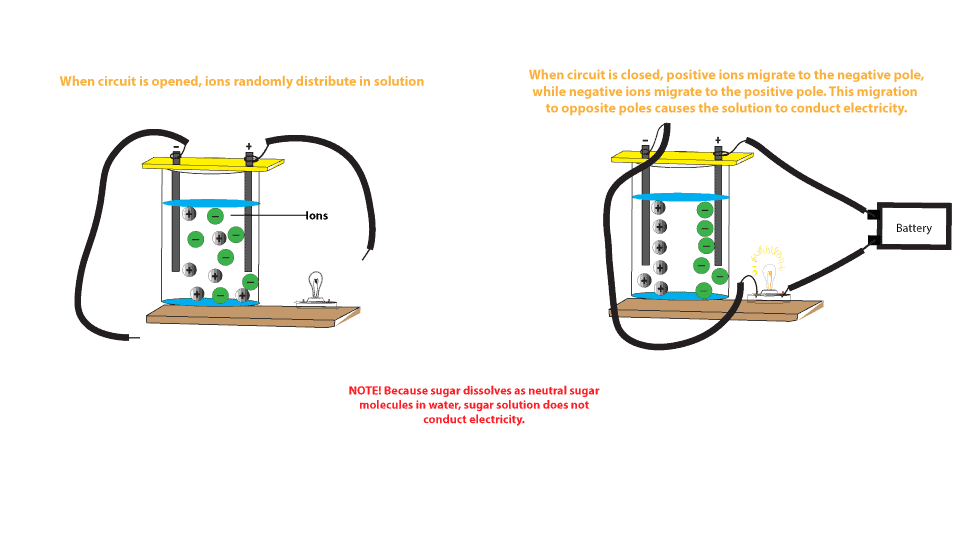
How does water dissolve salts - Florida State College at ... These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes. Please Watch animation 10.3 on ionic solutions.
Seawater - Wikipedia Seawater, or salt water, is water from a sea or ocean.On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/l, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na +) and chloride (Cl −) ions).Average density at the surface is 1.025 kg/l.
Solubility Diagram - ScienceGeek.net Solubility Diagram. Show all questions. 1 / 12. At approximately what temperature does the solubility of sodium chloride, NaCl, match the solubility of potassium dichromate, K 2 Cr 2 O 7? 83 ºC. 60 ºC. 50 ºC. 30 ºC. Which salt is LEAST soluble at 0 ºC?
What dissolves in salt ionic? - JacAnswers What dissolves in salt ionic? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.




![Solved [5 Points] 4. With reference to the solubility of ...](https://media.cheggcdn.com/media%2F7d5%2F7d58c908-67e9-4713-8bd3-704a3fa8296c%2FphpKFyimU.png)













0 Response to "36 diagram of salt dissolved in water"
Post a Comment