37 No+ Mo Diagram
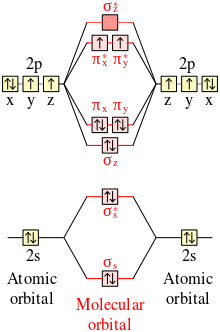
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine 37 no+ molecular orbital diagram - Diagram For You No+ molecular orbital diagram Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals.
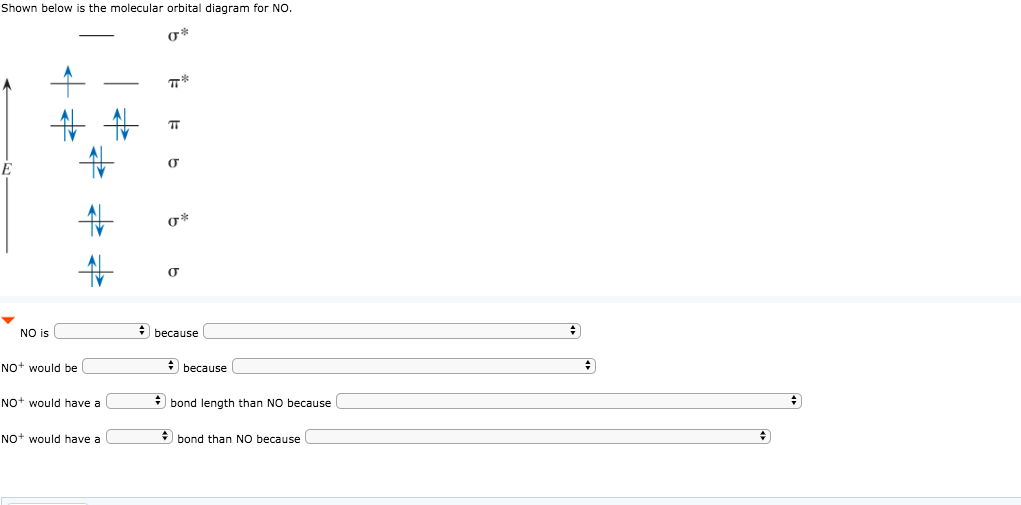
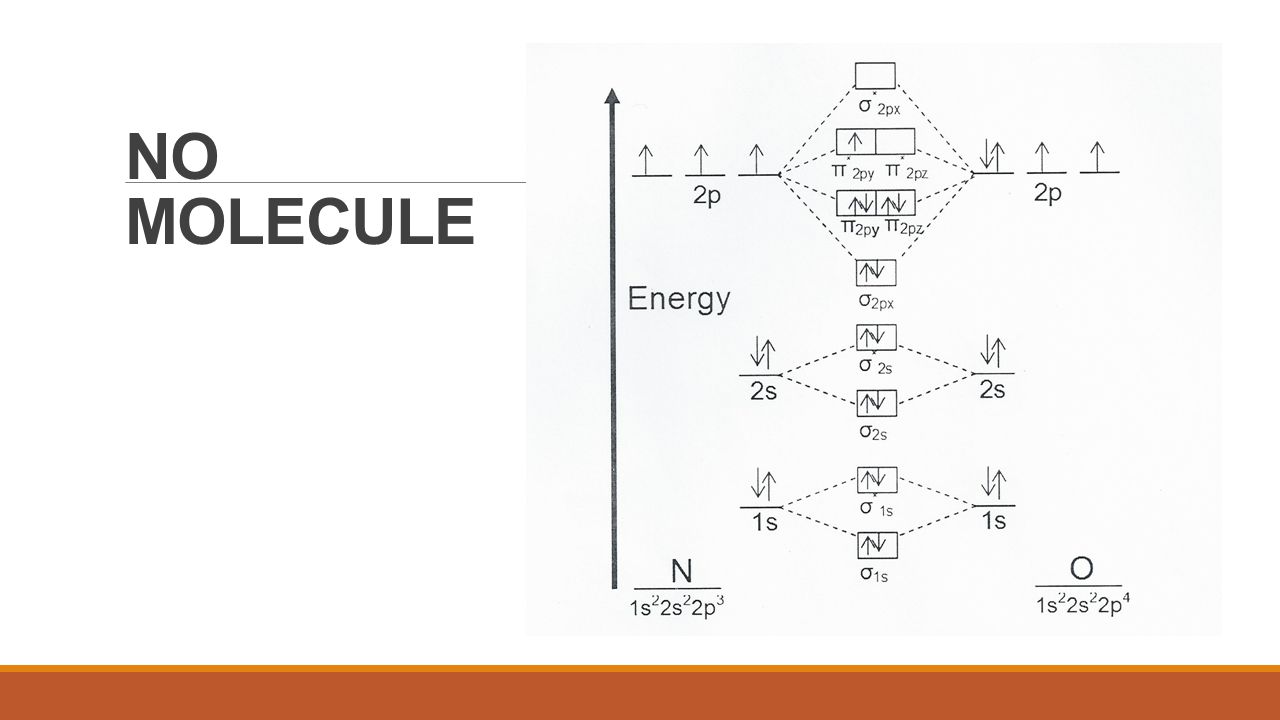
Solved 1a) Draw the molecular orbital diagram of NO, place ... 1a) Draw the molecular orbital diagram of NO, place the electrons in the atomic and molecular orbitals, label the atomic and molecular orbitals. 1b) How many electrons are placed in the π* orbitals of NO, NO+ and NO-1c) Determine the spin states S for NO, NO+ and NO- . two spin states may exist for NO-, what are they?
No+ mo diagram
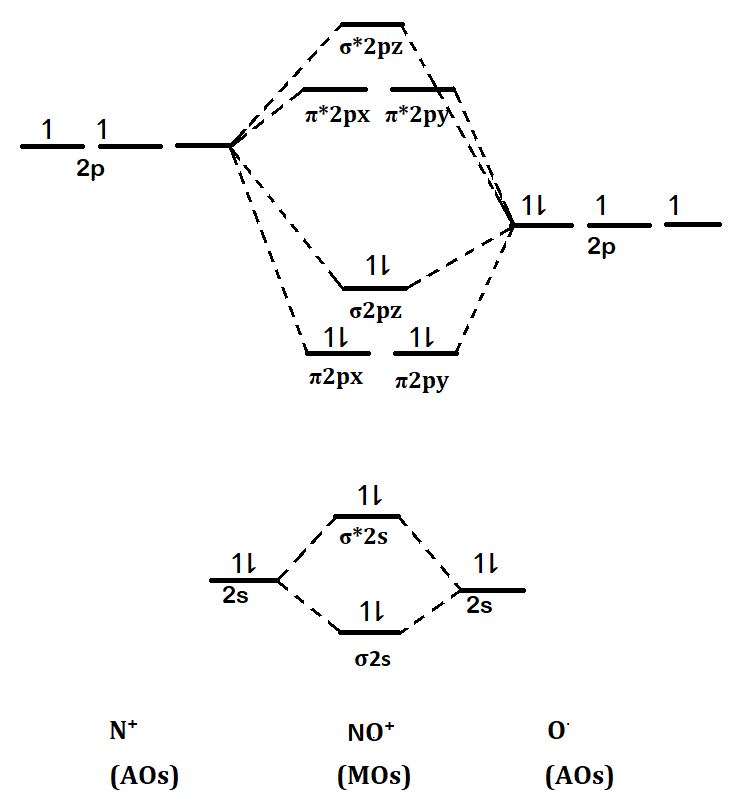
PDF Answer on the question #54656 Chemistry General chemistry The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding electrons and the number of antibonding electrons, divided by two. Draw the molecular energy level diagrams of NO+, NO, and ... Answer to: Draw the molecular energy level diagrams of NO+, NO, and NO-. Calculate their bond orders and give their magnetism (diamagnetic or... What is the bond order of NO and NO+? Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Bondorder = 1 2[a-b] B o n d o r d ...
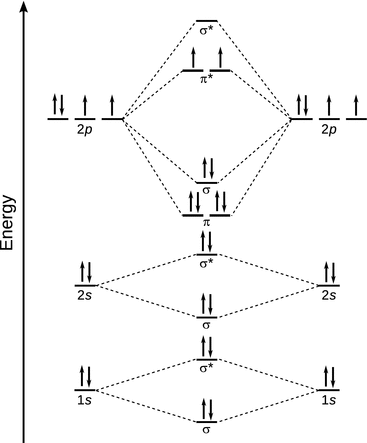
No+ mo diagram. Use the molecular orbital diagram shown to... | Clutch Prep Construct the molecular orbital diagram of NO+ and calculate for the bond order and determine if its paramagnetic or diamagnetic. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Determine if there's an unpaired MO (paramagnetic or diamagnetic) Explain the MO diagram for NO molecule. - Sarthaks ... Explain the MO diagram for NO molecule. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 23, 2020 by Taashi (15.8k points) selected Dec 24, 2020 by Aashi01 . Best answer. 1. Electronic configuration of N atom is 1s 2 2s 2 2p 3 . 2. ... Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Molecular Orbital Diagram Of NO,NO+ And HCl Molecules ... Lecture On Molecular Orbital Diagram Of NO,NO+ And HCl Molecules Which Is Explained In Details..Molecular Orbital Energy Level Diagramhttps://youtu.be/Go6VyX...
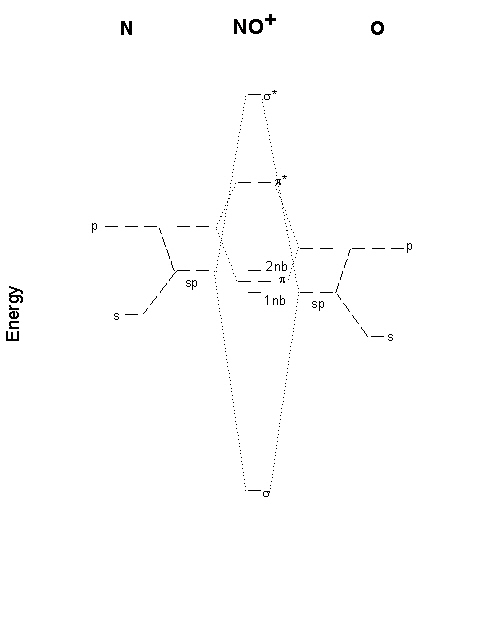
Using the MO diagram of "NO", calculate the bond order ... The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ... Consider the following molecules: NO, NO+ and NO-. Using ... Answer (1 of 5): NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 valence electrons: Same as NO+ but add (Pi*2px,Pi*2py)^1 NO- has 12 valence electrons: Same as NO but change ^1 at the end to ^2. Your MO diagram for NO should look like this: O is m... Answered: Draw the molecular orbital diagrams for… | bartleby Draw the molecular orbital diagrams for NO, NO+, and NO-. For each molecule, determine the bond order, if the molecule is stable, and if the molecule is stable if it is paramagnetic or diamagnetic. Rank the molecules in increasing order of bond strength. What is the molecular orbital diagram for NO? - Quora Answer (1 of 2): This image shows the molecular orbitals of nitric oxide and the types of bonds present.
Solved: Draw the molecular orbital diagrams for NO- and ... 115E Draw the molecular orbital diagrams for NO - and NO +. Compare the bond orders in these two ions. Step-by-step solution Step 1 of 5 Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Chapter 10, Problem 115E is solved. 5.3: Heteronuclear Diatomic Molecules - Chemistry LibreTexts 5.3: Heteronuclear Diatomic Molecules. Diatomic molecules with two non-identical atoms are called heteronuclear diatomic molecules. When atoms are not identical, the molecule forms by combining atomic orbitals of unequal energies. The result is a polar bond in which atomic orbitals contribute unevenly to each molecular orbital. Cyanide Molecular Orbital Diagram - schematron.org MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in carbon is six. Nitrilooxonium | NO+ - PubChem Nitrilooxonium | NO+ - PubChem. National Center for Biotechnology Information. 8600 Rockville Pike, Bethesda, MD, 20894 USA. Contact. Policies. FOIA. National Library of Medicine. National Institutes of Health. Department of Health and Human Services.
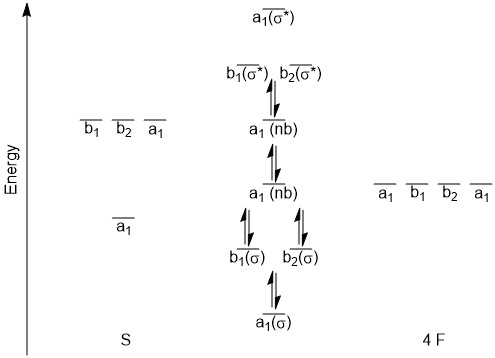
According to the molecular orbital energy-level diagram ... Answers: 3 on a question: According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO+, and NO−? These molecular orbitals are formed from the 2s and 2p atomic orbitals.
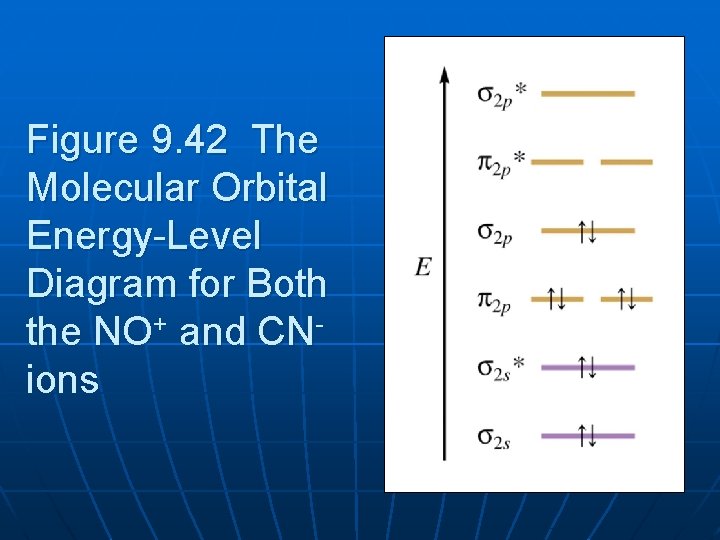
Problem+set+4 KEY CHEM+4320 - CHEM 4320 Problem Set - StuDocu Molecule Bond order NO+ 3 NO 2. NO- 2. A 1 T 2. Construct of MO diagram for NF. Assume s/p mixing similar to homonuclear diatomics of the 2nd row before O 2. The MO diagram of NF is shown below. What happens to the orbital overlap and, hence, energy of the MO, as the bond angle for the MOs shown below is increased toward 180 degrees?
energy - Molecular orbital diagram for nitrogen monoxide ... For this reason, I am reluctant to present a molecular orbital diagram. However, I will provide calculated MOs. While it may be tempting to just look at the pictures, the rationalisation behind it is more important. What will follow now are a few attempts at that rationalisation. In the nitric oxide series, $\ce{NO+}$ is surprisingly the simplest.
MO diagram for NO+ - CHEMISTRY COMMUNITY Re: MO diagram for NO+. Postby Chem_Mod » Mon Nov 21, 2011 11:19 pm. Just draw the atomic orbitals of O lower. This will result in the antibonding molecular orbitals being closer to N. Top. 2 posts • Page 1 of 1. Return to "*Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)". Jump to.
What is the bond order of NO+ class 12 chemistry CBSE Molecular orbital diagram shows which electrons are present in bonding orbitals and which are present in antibonding orbitals. - In some cases, we get bond order as a fractional number also. Let's see how much electrons are present in antibonding orbitals and bonding orbitals of \[N{{O}^{+}}\] in order to determine the bond order by MO diagram.
write M.o.T of No+.calculate its bond order. - Brainly.in Your MO diagram for NO should look like this: O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on O. Note the odd electron is in a Pi*2p orbital. Now draw two more MO diagrams for NO+ and NO-
MOT(VI)NO, NO+&NO- - YouTube ¤ Molecular Orbital diagram of BN, CN, CN- ¤Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤ S-P mixing OF ORBITALS ...
What is the bond order of NO and NO+? Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Bondorder = 1 2[a-b] B o n d o r d ...
Draw the molecular energy level diagrams of NO+, NO, and ... Answer to: Draw the molecular energy level diagrams of NO+, NO, and NO-. Calculate their bond orders and give their magnetism (diamagnetic or...
PDF Answer on the question #54656 Chemistry General chemistry The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding electrons and the number of antibonding electrons, divided by two.












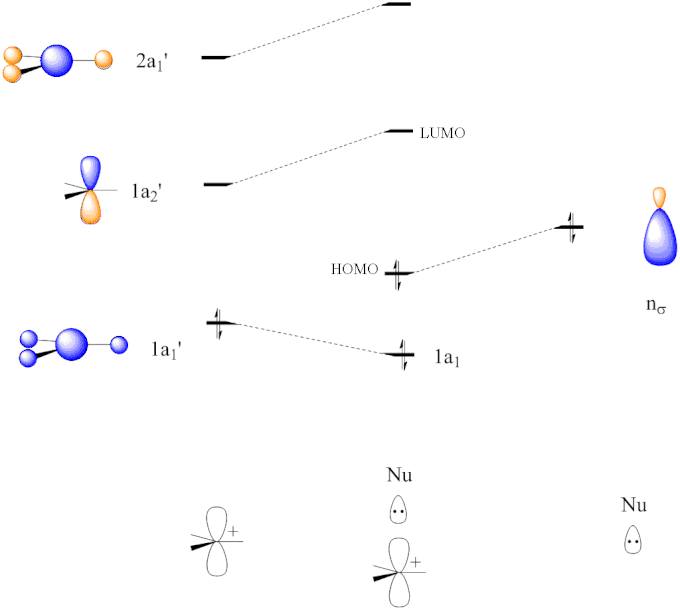




0 Response to "37 No+ Mo Diagram"
Post a Comment