38 phase change diagram endothermic exothermic
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. PDF Exothermic vs endothermic reaction graphs The extra energy is released to the surroundings.Reactants --> Products + EnergyActivation Energy "Ea"-The Energy required to initiate a chemical reaction. Both endothermic and exothermic reactions require activation energy.Endothermic Reactionsthe reactants have less potential energy than do the products.
Solved Classify the following phase changes as exothermic ... Transcribed image text: Classify the following phase changes as exothermic processes or endothermic processes. Drag the appropriate items to their respective bins.
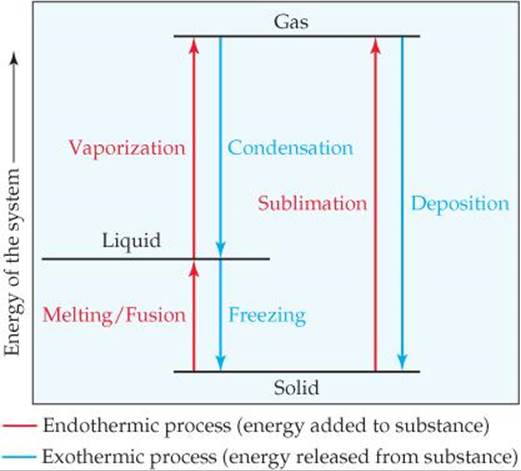
Phase change diagram endothermic exothermic
Phase Changes: Exothermic or Endothermic? - YouTube Phase Changes: Exothermic or Endothermic? If playback doesn't begin shortly, try restarting your device. Videos you watch may be added to the TV's watch history and influence TV recommendations. Energy Diagrams of Reactions | Fiveable If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings. Phase Changes endothermic and exothermic phase changes Questions and ... endothermic and exothermic phase changes. STUDY. PLAY. endothermic. absorbs energy. exothermic. releases energy. melting. endothermic.
Phase change diagram endothermic exothermic. Solved Phase diagrams help us to visualize how changes in ... Phase diagrams help us to visualize how changes in temperature and pressure affect a substance's state of matter. We can also use the information embedded within a phase diagram to help us think about whether a phase change is endothermic or exothermic. The following questions refer to this phase diagram and transitions between positions on the ... Endothermic and Exothermic Processes - Kentchemistry.com Exothermic Processes: Endothermic Processes: freezing water; solidifying solid salts; condensing water vapor; making a hydrate from an anhydrous salt; forming an anion from an atom in the gas phase; Annihilation of matter E=mc 2; splitting of an atom : melting ice cubes; melting solid salts; evaporating liquid water; making an anhydrous salt from a hydrate Endothermic Reaction | Characteristics, Examples ... Learn the process of endothermic reactions. Explore the definition, characteristics, examples, equations, and energy level diagrams of endothermic reactions as well as a comparison with exothermic ... What 3 phase changes are exothermic? - FindAnyAnswer.com Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. Click to see full answer
PDF Chapter 3 States of Matter Section 3.3 Phase Changes Endothermic Exothermic Characteristics of Phase Changes (pages 84-86) 1. What is a phase change? Match each term with the letter of the phase-change description that best describes it. Term Phase-Change 2. freezing a. Solid to gas 3. sublimation b. Liquid to gas 4. condensation c. Gas to solid 5. melting d. Liquid to solid 6. deposition e. Gas to liquid States of Matter / Phase Changes Notes - Google Docs States of Matter Notes State of Matter (name and drawing of particles) Describe Particles Properties (indefinite vs. definite shape/volume) Solid Liquid Gas Plasma Phase Change Diagram Label endothermic phase changes in red and exothermic phase changes... Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions. How do you know if a phase change is endothermic or ... What phase change is endothermic? Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system.
Is Sublimation Endothermic or Exothermic? - Techiescientist Sublimation is a phase transition process wherein a substance converts from a solid to a gas without forming an intermediate liquid state. It is an endothermic process because the energy in the form of heat is required to break the intermolecular forces of attraction present between the molecules of the solid to convert it into a gas. Phase Changes - Exo & Endo Flashcards | Quizlet Pick out the exothermic and endothermic phase changes Learn with flashcards, games, and more — for free. What Are Some Exothermic and Endothermic Phase Changes? An endothermic phase change absorbs heat or energy, while an exothermic phase change releases heat or energy, states the Washington University Department of Chemistry. The following are additional endothermic phase changes: Fusion, or melting, when a solid transforms to liquid. Vaporization, when a liquid transforms to gas. Phase diagrams (video) | Thermochemistry | Khan Academy This is the phase diagram for water. So just to understand what's going on here, is that on this axis, I have pressure. On the x-axis, I have temperature, and at any given point, this diagram will tell you whether you're dealing with a solid, so solid will be here, a liquid will be here, or a gas.
Is Sublimation Endothermic Or Exothermic Process? Is Sublimation Endothermic Or Exothermic. Sublimation has also been applied as a generic term to describe a solid-to-gas transition (sublimation) pursued by a gas-to-solid transition (deposition). While vaporization from liquid to gas appears as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with the construction of bubbles in the interior of ...
Phase Changes Exothermic Or Endothermic - Dubai Burj Khalifas What 3 phase changes are exothermic? fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. changes of state are examples of phase changes, or phase transitions. all phase changes are accompanied by changes in the energy of a system.
DOC Phase Changes Worksheet - Boyertown Area School District Label this on the diagram. (40.7 kJ per mol for water) Phase Change Diagram. The graph was drawn from data collected as 1 mole of a substance was heated at a constant rate. Use the graph to answer the following questions. Use the phase change diagram above to answer the following questions. Describe what is occurring from; A to B. B to C. C to D. D to E
What phase changes are exothermic and endothermic ... Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase (Table 4.8, Fig. 4.2). Sublimation is an endothermic phase transition that occurs at temperatures and pressures below the triple point of a chemical in the phase diagram.
Endothermic Reaction Coordinate Diagram A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism.
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Difference Between Endothermic and Exothermic Reactions ... The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more. Difference Between Endothermic and Exothermic Reactions
Changes of State When attempting to understand the phase changes it is important to remember what is occurring in endo and exothermic reactions. Endothermic - Energy is being absorbed - bonds are breaking - products are less ordered Exothermic - Energy is being released - bonds are being formed - products are more ordered
Exothermic and endothermic reactions - Energy changes in ... The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This can be used to classify reactions as exothermic or endothermic.
How to Determine if Phase Change is Endothermic or ... 📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at 💯 If you like my teaching style and are inte...
Exothermic, Endothermic, & Chemical Change | Energy ... Identifying Exothermic & Endothermic Reactions. There are two methods for distinguishing between exothermic and endothermic reactions. Monitor temperature change When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature decreases.
endothermic and exothermic phase changes Questions and ... endothermic and exothermic phase changes. STUDY. PLAY. endothermic. absorbs energy. exothermic. releases energy. melting. endothermic.
Energy Diagrams of Reactions | Fiveable If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings. Phase Changes
Phase Changes: Exothermic or Endothermic? - YouTube Phase Changes: Exothermic or Endothermic? If playback doesn't begin shortly, try restarting your device. Videos you watch may be added to the TV's watch history and influence TV recommendations.

0 Response to "38 phase change diagram endothermic exothermic"
Post a Comment