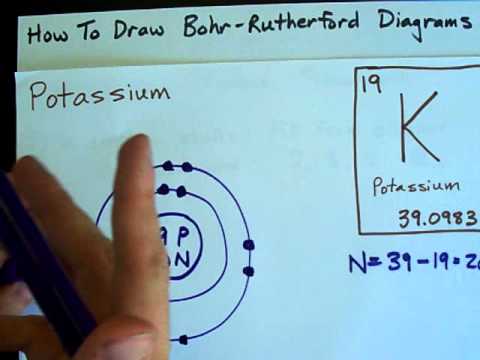
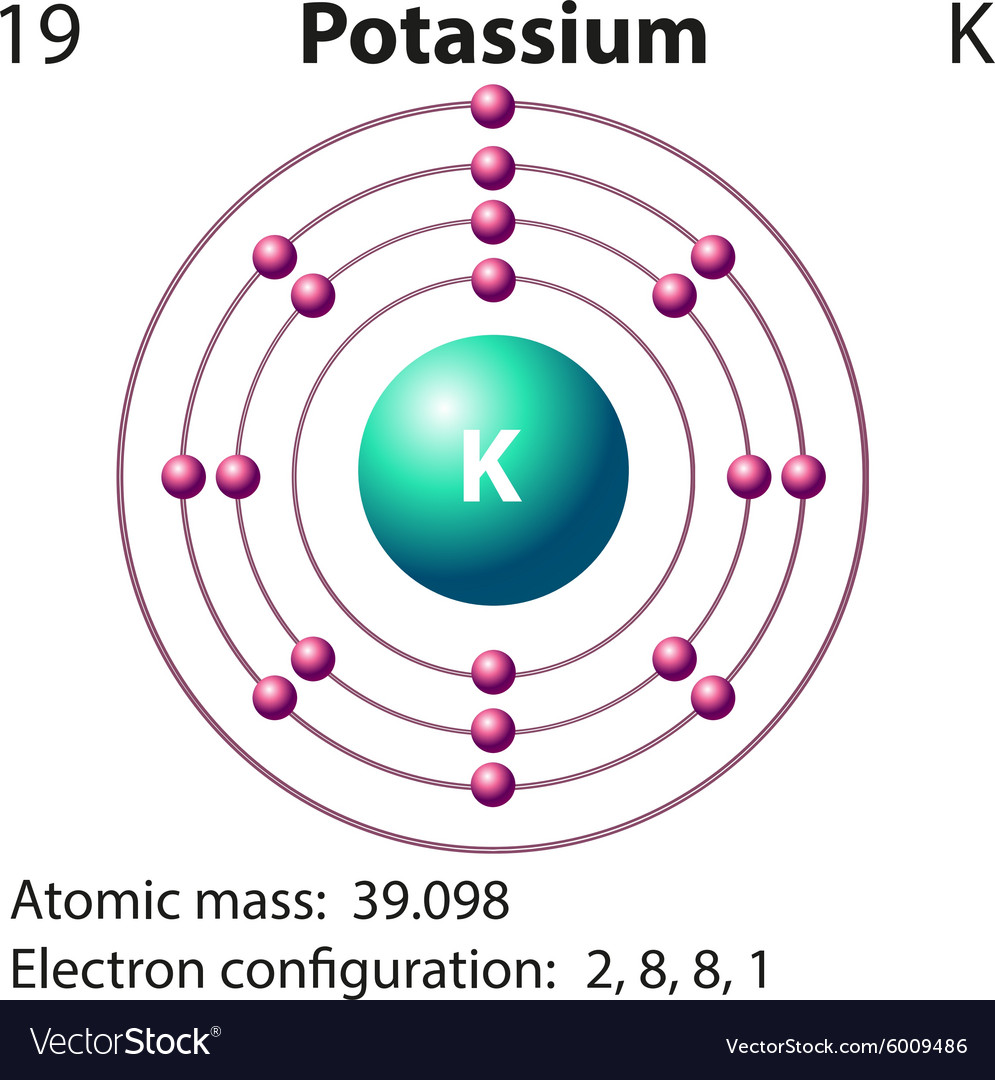
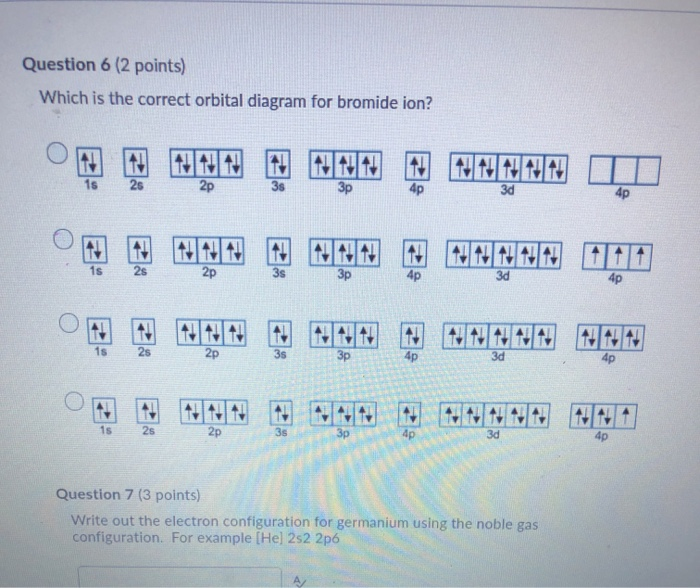
39 select the correct orbital diagram for this element potassium
Orbital Diagrams Flashcards | Quizlet The orbital diagram represents what element? (1s2 2s2 2p6 3s2 3p6 4s) Potassium. What atom will have unpaired 2p electrons? O (oxygen) ... Which of the elements below would require special attention to this rule to correctly depict the orbital diagram? N (nitrogen) Related questions. What is the correct electron configuration for potassium ... What is the correct electron configuration for potassium (A=19)? A. 1s 2 2s 2 2p 6 3s 3 3p 6 B. 1s 2 2s 2 2p 6 3s 2 3p 7 C. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 D. 1s 2 2s 2 2p 6 3s 2 3p 5 4s 2. 8. The abbreviated electron configuration for arsenic is: A. [Ar]4s 2 4p 3 B. [Ar]4s 2 4d 10 4p 3 C. [Ar]4s 2 3d 10 4p 3 D. [Zn]4p 3.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below.

Select the correct orbital diagram for this element potassium
How do you write the orbital diagram for carbon? | Socratic The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ... Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Select the correct orbital diagram for this element ... diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. How many orbitals does potassium have? Potassium has 4 orbitals. therefore, potassium has 19 electrons. Orbital 1...
Select the correct orbital diagram for this element potassium. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. Chem300 quiz 10.docx - 1. Select the correct number of ... Select the correct number of valence electrons for each element. a. sodium b. oxygen c. xenon d. copper a. 1 b. 6 c. 8 d. 11 2. Order the elements from lowest electronegativity to highest electronegativity. (Hint: use the provided electronegativity diagram) oxygen, chlorine, potassium, hydrogen a) potassium, hydrogen, chlorine, oxygen 3. Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ... Hund's Rule and Orbital Filling Diagrams | Chemistry for ... According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the 2s.. The filling diagram for carbon is shown in the Figure below.
Solved 20. Select the correct statement below: a) | Chegg.com This problem has been solved! 20. Select the correct statement below: a) Phosphorous contains 10 core electrons and 5 valence electrons. Its orbital diagram contains one half-filled 3p orbital and two filled 3p orbitals. b) Aluminum contains 10 core electrons and 3 valence electrons. 02/16 Super Tic Tac Toe Jeopardy Template Determine if the following electron configurations are correct: a) 1s 2 2s 2 2p 6 3s 2 3p 6 4s ... can only be 2 electrons in an s-orbital. 400. Write the abbreviated electron orbital diagram for the following element: Germanium *Go to slides for answer* 400. Write the unabbreviated electron configurations of the following elements: a ... Periodic Table and Electron Configuration Quiz - Quizizz answer choices. states that single electrons electrons with the same spin must occupy each-energy orbital before additional electrons with opposite spins can occupy the same orbitals. states that each electron occupies the lowest energy orbital available. states that a maximum of two electrons can occupy a single atomic orbital, but if only if ... Chemistry: Test #4 - Ch. 8, 9, 10 Flashcards | Quizlet Which is the correct orbital diagram for vanadium? Look at pg. 333. n=4, l=1, m1= 0, ms = 1/2 ... Choose the element with the highest first ionization energy from each pair. A. Br or Bi B. Na or Rb C. As or At ... The relative size of sodium and potassium ions is an example of a _____ _____: one that is predictable based on an element's ...
Electron Configuration Jeopardy Template Electron Configuration. No teams 1 team 2 teams 3 teams 4 teams 5 teams 6 teams 7 teams 8 teams 9 teams 10 teams Custom. Press F11. Select menu option View > Enter Fullscreen. for full-screen mode. Edit • Print • Download • Embed • Share. JeopardyLabs. Chemistey Ex 2 Flashcards | Quizlet Which of the following statements correctly describe how an orbital diagram is constructed? Select all that apply. ... Select the correct condensed electron configuration for the element S (Z = 16). [Ne]3s23p4 ... Consider the formation of an ionic bond between the elements potassium (K) and chlorine (Cl). Which of the following statements ... What is the orbital diagram of the element gold? - Answers The element is Cobalt (Co)Cobalt, which is classified as a metal, has 27 electrons in its orbital diagram. Its chemical symbol is Co and its atomic number is 27.Cobalt. Exam 2 Intro to Chem Flashcards | Quizlet When completing the orbital diagram for the element phosphorus, which of the following statements is correct? A. There are five electrons in the n=3 energy B. The 2p sublevel is not full C. There are electrons in the 3d sublevel D. There are no electrons in the 3s sublevel E. There is one unpaired electron in the 3p sublevel
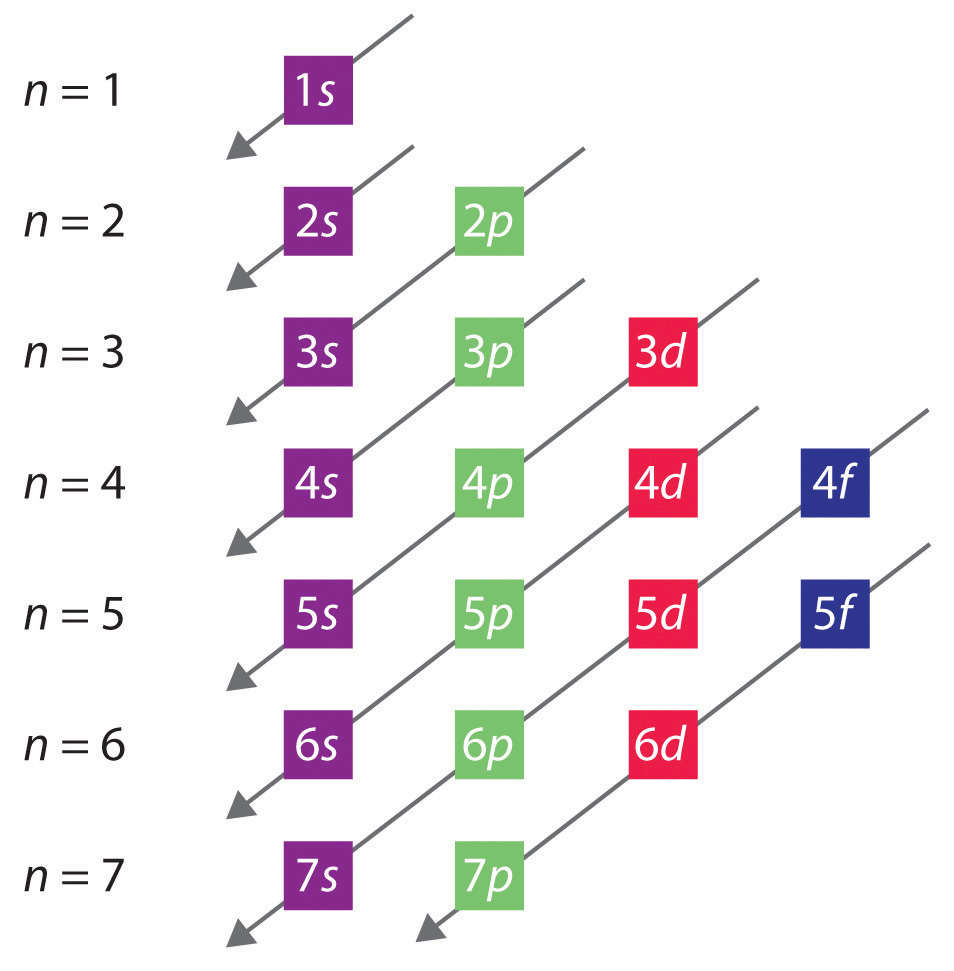
6.4 Electronic Structure of Atoms (Electron Configurations ... The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3 s orbital, giving a 1 s 2 2 s 2 2 p 6 3 s 1 configuration.
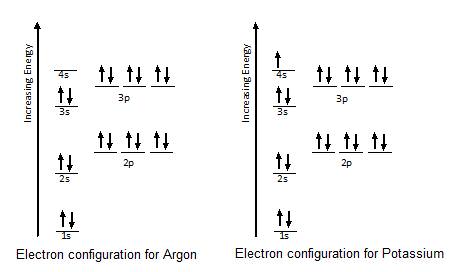
Solved The orbital diagram provided represents what ... The orbital diagram provided is of potassium.We know that potassium is the 19th element on the periodic table.Electrons fill orbitals… View the full answer Transcribed image text : The orbital diagram provided represents what element? ft | ft It It It It | IL LIL | 1s 2s 2p 3s 3p 4s Select the correct answer below: O potassium sodium O ...
What is the correct set of quantum numbers (n, l, ml, ms ... Here's what I got. Your starting point here will be tin's electron configuration. Tin, "Sn", is located in period 5, group 14 of the periodic table and has an atomic number equal to 50. This tells you that a neutral tin atom will have a total of 50 electrons surrounding its nucleus. So, the electron configuration for tin looks like this - I'll use the noble gas shorthand notation "Sn: " ["Kr ...
Ch.2: Atoms and the Periodic Table Flashcards - Quizlet Arsenic. An element is a pure substance that cannot be broken down by a _________ process. chemical. Match each structural feature of the periodic table with its definition: group. - A vertical column of elements in the periodic table. - A horizontal row of elements in the periodic table. A vertical column of elements in the periodic table.
Electron Configuration for Sulfur (S) - UMD In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital.
Orbital Diagram For Vanadium (V) | Vanadium Electron ... Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free of cost and no charges are ...
Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
Electron Configuration for Potassium (K) In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Select the correct orbital diagram for this element ... diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. How many orbitals does potassium have? Potassium has 4 orbitals. therefore, potassium has 19 electrons. Orbital 1...
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
How do you write the orbital diagram for carbon? | Socratic The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
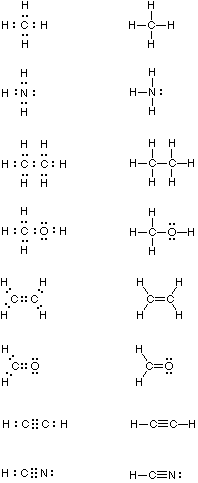
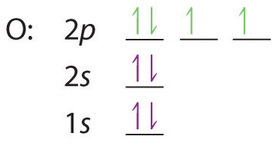
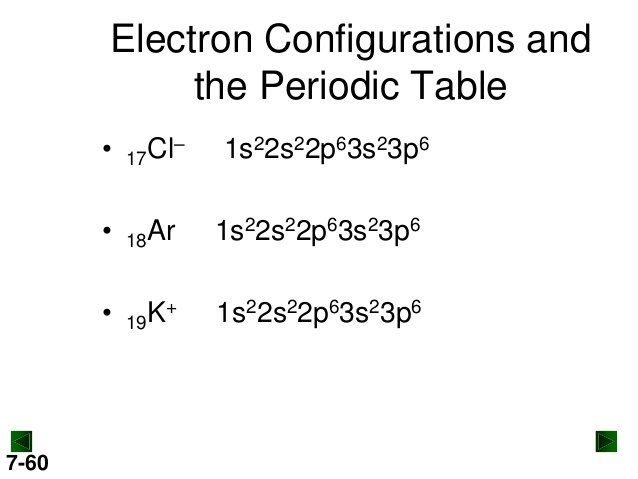

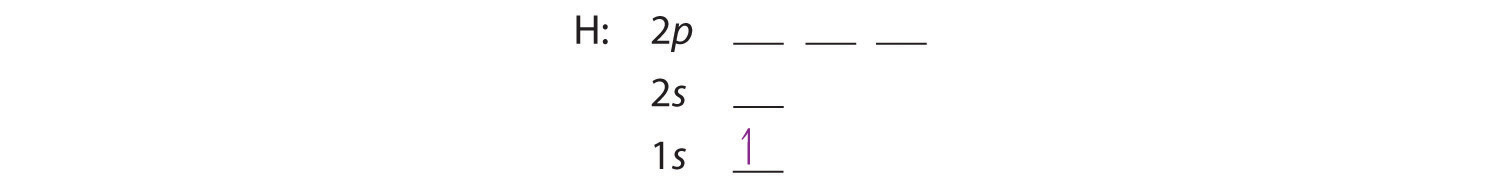












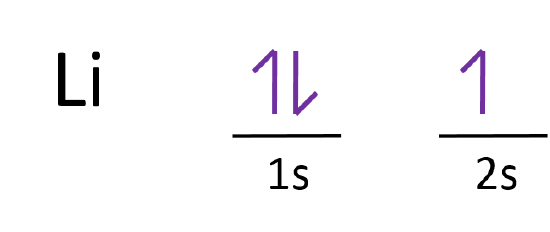








0 Response to "39 select the correct orbital diagram for this element potassium"
Post a Comment