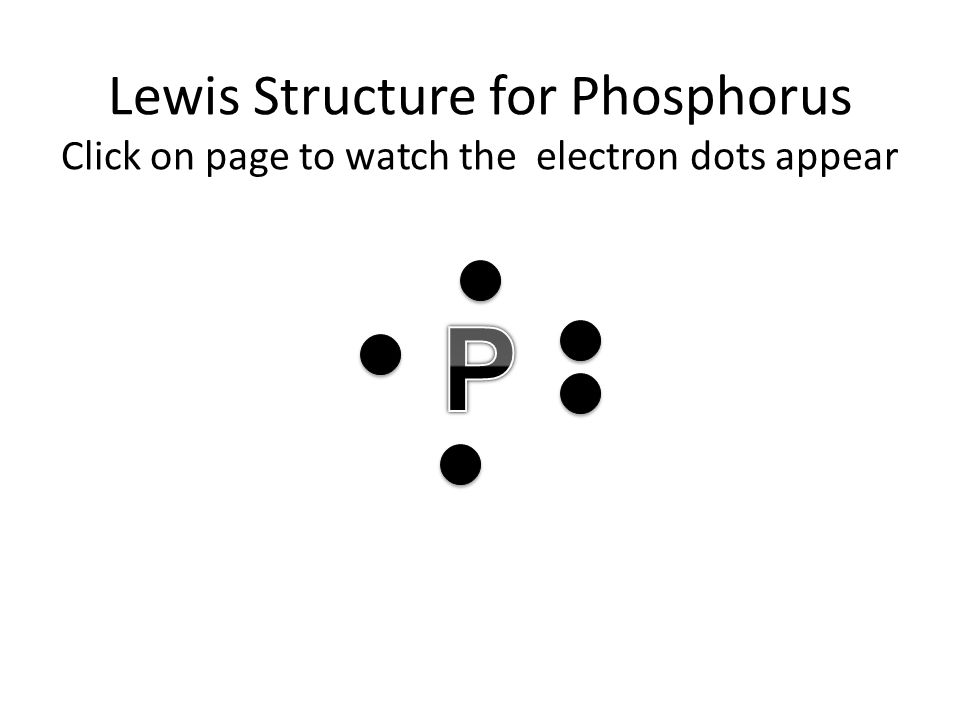
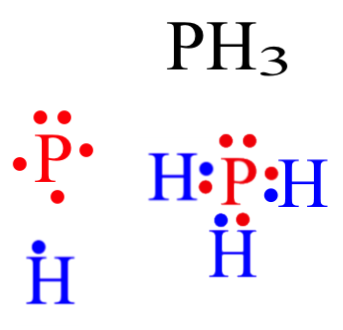
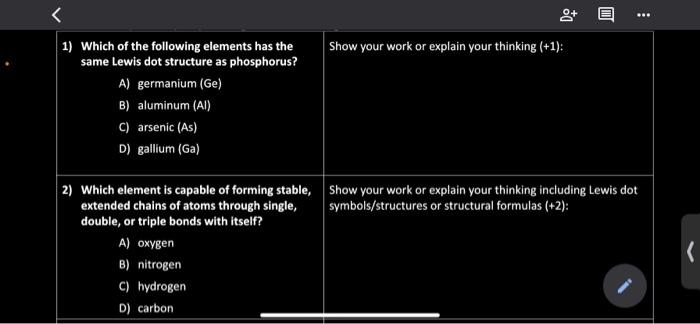
37 electron dot diagram for phosphorus
PBr3 Lewis Structure, Molecular Geometry, Polarity, and ... Phosphorus tribromide or Pbr3 molecule consists of a phosphorus atom and three atoms of bromine. Phosphorus has an atomic number of 15 and therefore has a valency of 5. In the case of Br, it belongs to the family of halogens and consists of seven valence electrons. Total valence electrons in a single molecule of PBr3 = 5 + 7*3 = 5 + 21 = 26 PI3 Lewis Structure: How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the PI3 Lewis Dot Structure (Phosphorus triiodide).For the PI3 structure use the periodic table to find the total n...
How to Draw the Lewis Dot Structure for P 3 ... - YouTube A step-by-step explanation of how to draw the P3- Lewis Dot Structure.For the P3- Lewis structure use the periodic table to find the total number of valence ...
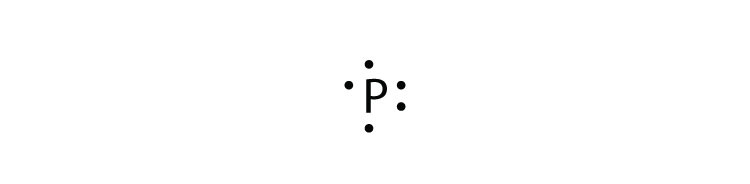
Electron dot diagram for phosphorus
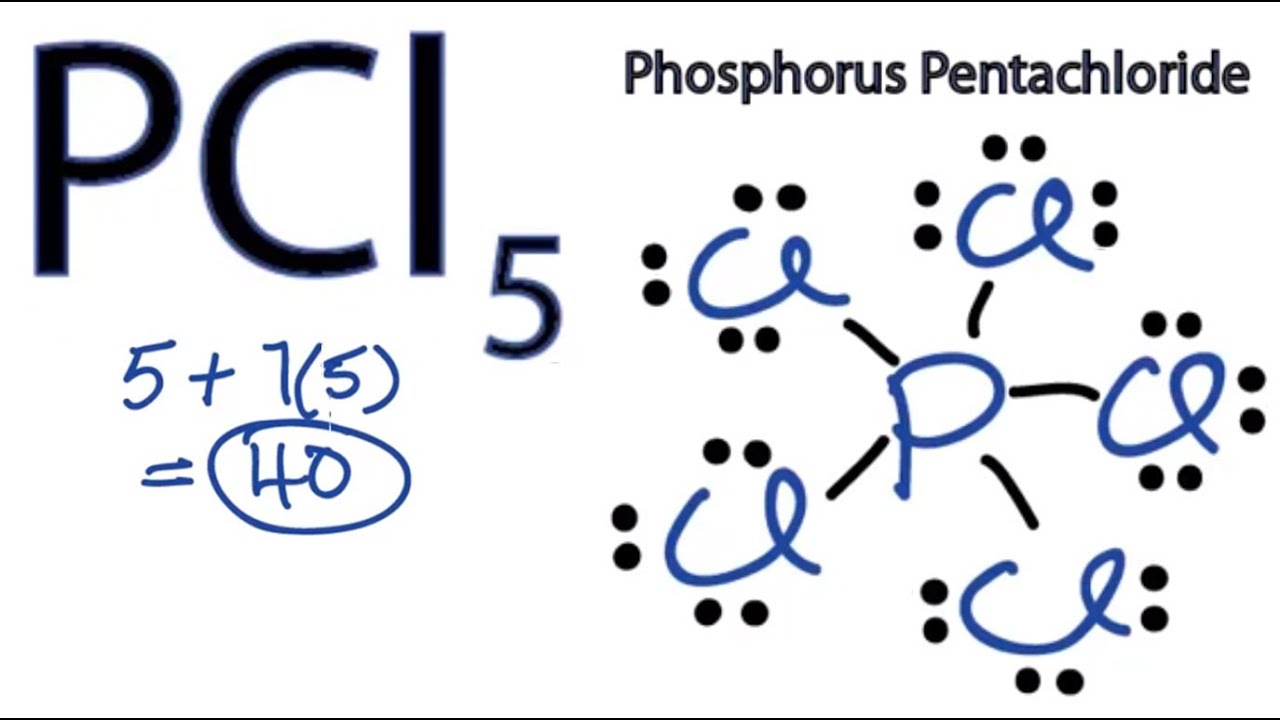
39 electron dot diagram for phosphorus Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Phosphorus, we got to know, it has 5 valence electrons. So, just represent the 5 valence electrons around the Phosphorus atom as a dot. What is the Lewis dot structure for P? - FindAnyAnswer.com The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s. PCl5 Lewis Structure, Molecular Geometry, Hybridization ... Drawing the Lewis structure of PCl5. Step 1: Count the number of valence electrons in a PCl5 molecule. We can refer to the periodic table for this. We come to understand that PCl5 is made up of Phosphorous and Chlorine. Phosphorus, having atomic number 15, has an electron composition of 2, 8, 5. Therefore, it has 5 electrons in its outermost shell.
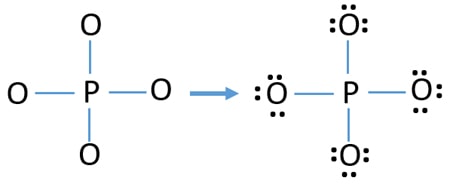
Electron dot diagram for phosphorus. PCl3 (Phosphorus Trichloride) Lewis Structure Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. valence electrons given by phosphorus atom = 5 * 1 = 5 valence electrons given by oxygen atoms = 7 * 3 = 21 What is the electron dot notation for phosphorus? - Answers It is K with one dot so: K . The reasoning behind this is that you put the highest energy level on the dot notation. Electron Configuration notation for Potassium is: 1s2; 2s2, 2p6; 3s2, 3p6, 4s1. PO43- Lewis Structure (Phosphate ion) Now all electron pairs are spent. There is no electron pairs to mark on phosphorous atom. Charges on atoms After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atom will get a -1 charge and phosphorous atom get a +1 charge. The overall charge of ion is ( -1*4 + (+1) ) = -3. PF3 lewis structure, Molecular geometry, Polar or nonpolar ... ⇒ Total valence electron in Phosphorous = 5 ⇒ Total valence electron in Fluorine = 7 ∴ Total valence electron available for drawing the PF3 lewis structure = 5 + 7*3 = 26 valence electrons [∴PF3 has three fluorine atom and one phosphorous] 2. Find the least electronegative atom and placed it at center
How to draw PCl3 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ... PBr3 lewis structure, molecular geometry, polar or ... PBr 3 lewis structure is made up of three P-Br bonds, with a phosphorus (P) atom in a central position and all three bromine (Br) as outer atoms in the lewis diagram. The lewis structure of PBr 3 contains a total of 3 bond pairs and 10 lone pairs(3 lone pairs on each bromine atom and 1 on the central atom).. The drawing of the PBr 3 lewis's structure is very easy and simple. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... PBr3 Lewis Structure, Molecular Geometry, Hybridization ... There are 26 valence electrons for Phosphorus Tribromide. PBr3 Lewis Structure Lewis dot structures or Lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. The valence electrons of atoms form bonds, and these bonds are represented by showing straight lines.
Lewis Dot Diagram For Phosphorus - schematron.org The DOT ID is also the UN Number">. More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. PF3 Lewis Structure, Molecular Geometry, and Hybridization PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition. Below are the steps to draw the lewis structure of the PF3 molecule 1. Find out the total number of valence electrons in PF3, which is 26. 2. Draw the electron dot symbol for a phosphorus atom. Which ... Draw the electron dot symbol for a phosphorus atom. Which statement below best describes the arrangement of electrons around the correct dot structure? a. three pairs of electrons Lewis Dot Structure for Phosphorous Atom (P) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for P (Phosphorous). I show you where Phosphorous is on the periodic table and how to dete...
Lewis Dot Diagram For Tellurium - schematron.org Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Lewis Dot Structure For Phosphorus Trichloride - drawing easy Lewis structure pcl3 molecular electron geometry, lewis structure, bond angles and hybridization posted by priyanka 07 feb phosphorus trichloride is made up of one phosphorus atom and three chlorine atoms, having a chemical formula of pcl3. Drawing pcl3 lewis structure is very easy to by using the following method. Source: kovodym.blogspot.com
P2O5 (Phosphorus pentoxide) Lewis Structure Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. valence electrons given by phosphorus atoms = 5 * 2 = 10 valence electrons given by oxygen atoms = 6 * 5 = 30
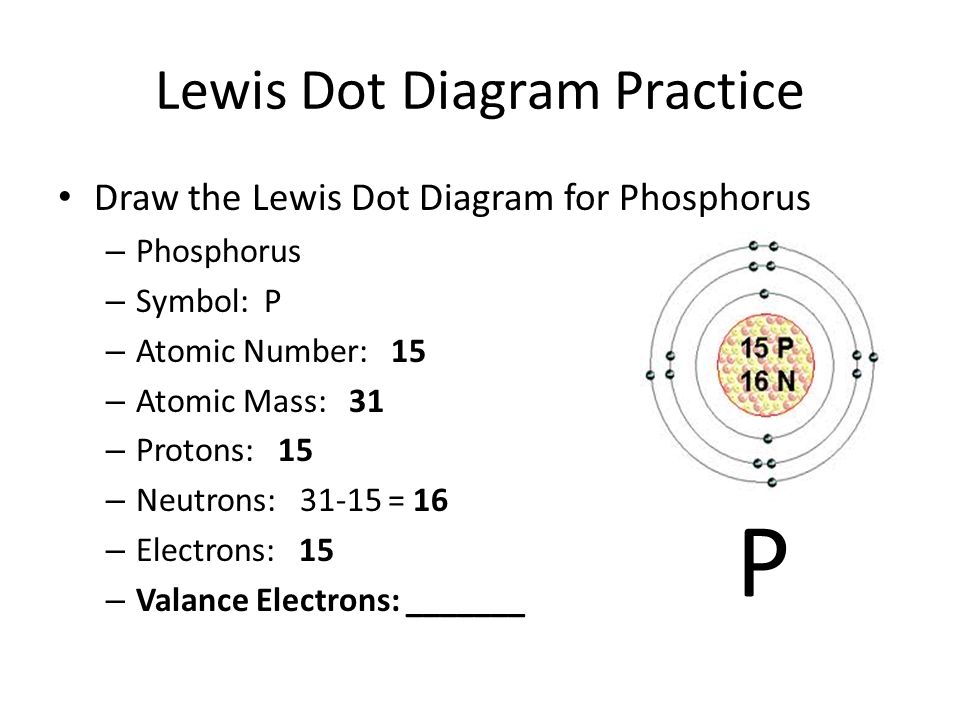
Electron Configuration for Phosphorus (P) - UMD The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Video: Phosphorus Electron Configuration Notation
Solved a) Draw a valid lewis electron dot Structure for ... Expert Answer 100% (14 ratings) (A) There are 26 valence electrons to be used when drawing the Lewis dot structure for Phosphorous trichloride; 5 from phosphorus as it occurs in the 15th column of the Periodic Table and 7 from each of the chlorine atoms as chlorine occurs in the 17 … View the full answer Previous question Next question
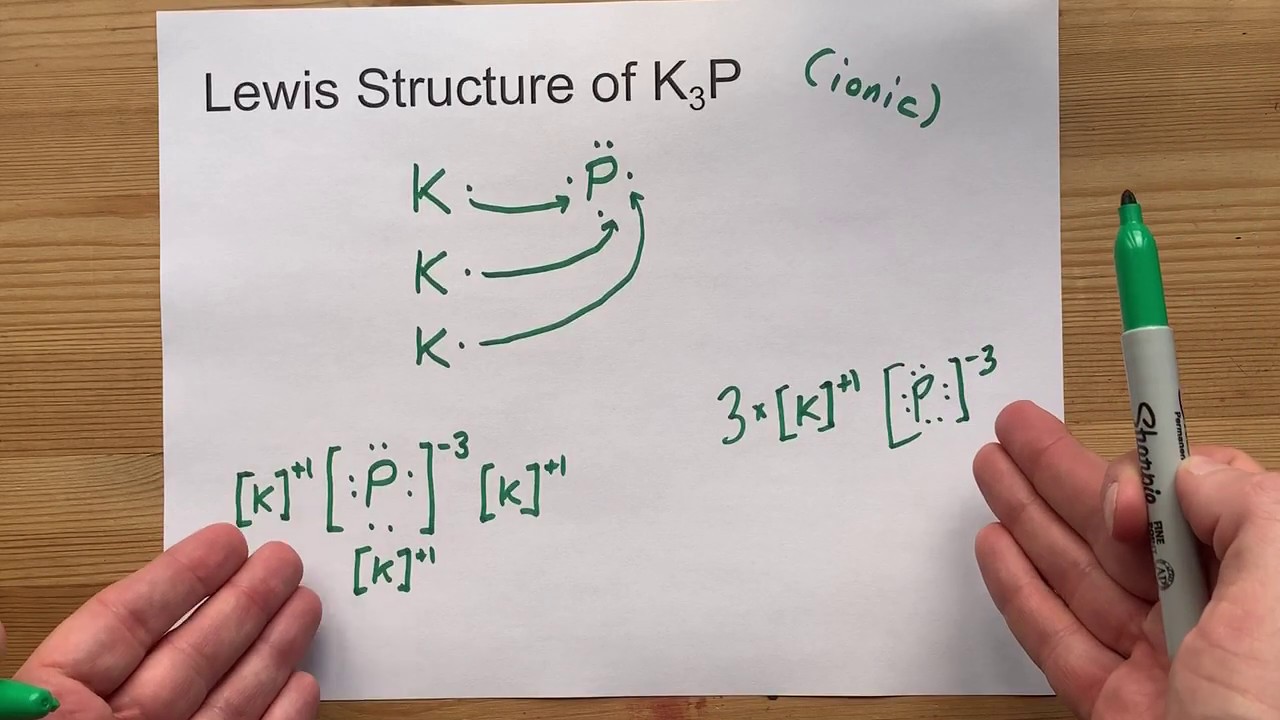
Lewis Electron Dot Diagrams - lardbucket Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, ... phosphorus; Draw the Lewis electron dot diagram for each element.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom 1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal.
PBr5 Lewis Structure, Molecular Geometry, Hybridization ... PBr5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. PBr5 or Phosphorous Pentabromide is a compound that consists of 5 molecules of Bromine and 1 molecule of Phosphorus. It appears to be a yellow crystalline solid. The structure of PBr5 in the solid-state is PBr4+ Br− whereas in the vapor phase it dissociates to become ...
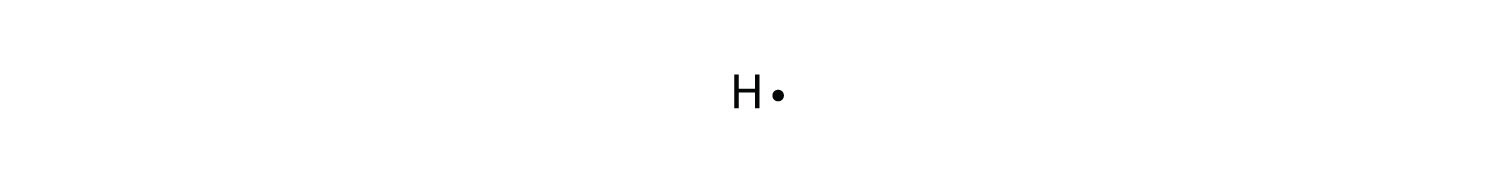
How would you draw a Lewis structure for an atom that has ... Explanation: Based on your electron configuration, the element that you want to draw is Phosphorus, since you have 15 electrons. Phosphorus has 5 valence electrons. To draw Lewis Structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it.
Lewis Dot Diagram Phosphorus - schematron.org Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since one of these electron pairs is a stereochemically active non-bonding pair, the. Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom.
PCl5 Lewis Structure, Molecular Geometry, Hybridization ... Drawing the Lewis structure of PCl5. Step 1: Count the number of valence electrons in a PCl5 molecule. We can refer to the periodic table for this. We come to understand that PCl5 is made up of Phosphorous and Chlorine. Phosphorus, having atomic number 15, has an electron composition of 2, 8, 5. Therefore, it has 5 electrons in its outermost shell.
What is the Lewis dot structure for P? - FindAnyAnswer.com The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s.
39 electron dot diagram for phosphorus Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Phosphorus, we got to know, it has 5 valence electrons. So, just represent the 5 valence electrons around the Phosphorus atom as a dot.




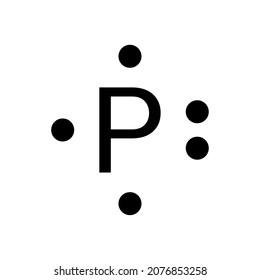



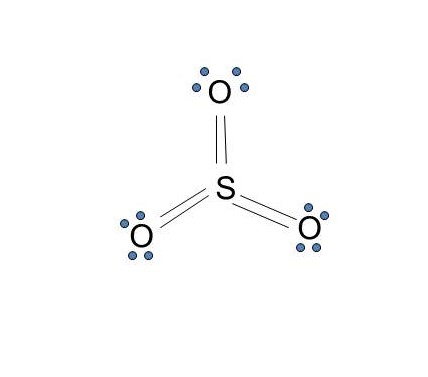









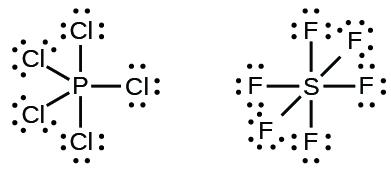



0 Response to "37 electron dot diagram for phosphorus"
Post a Comment