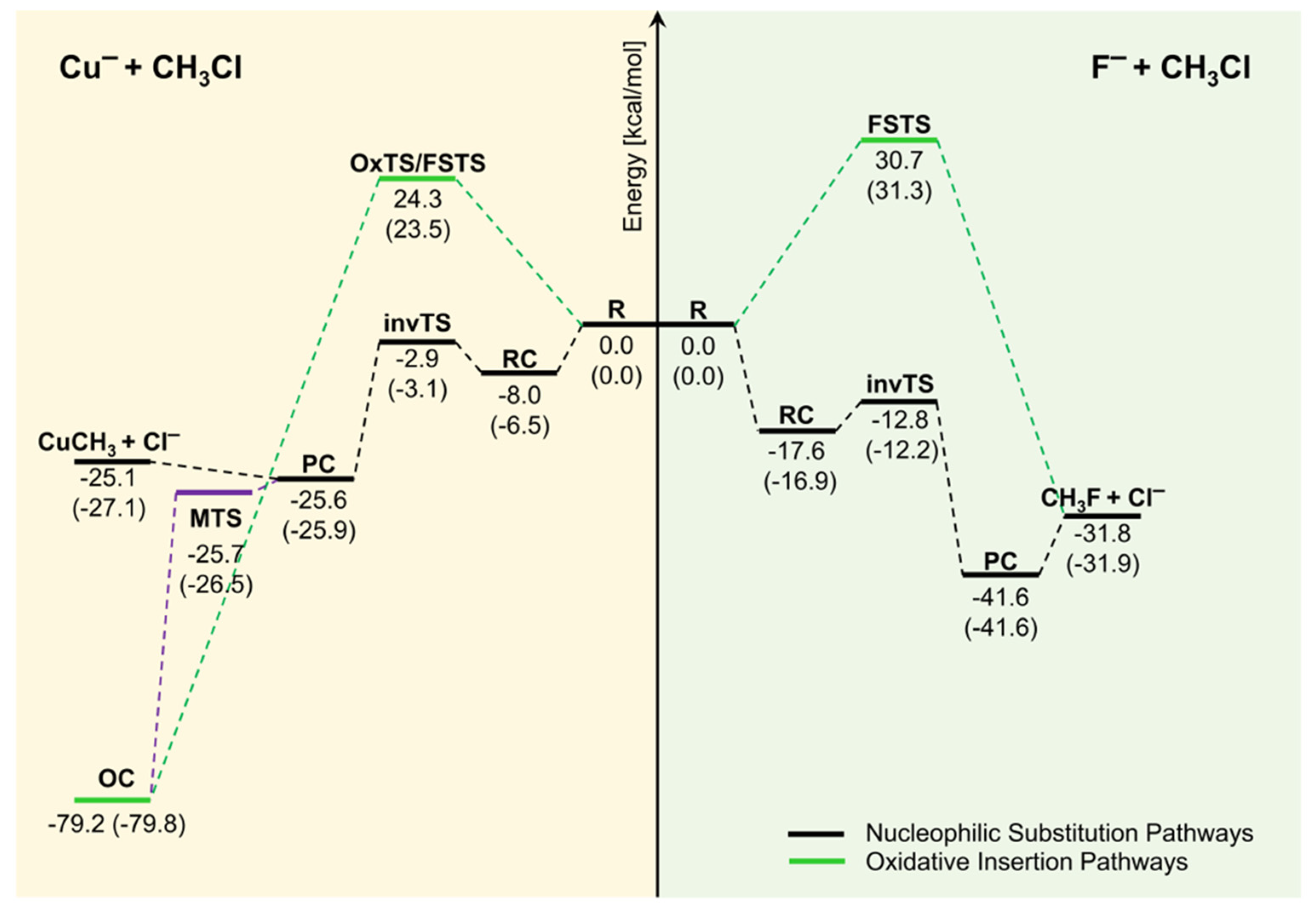
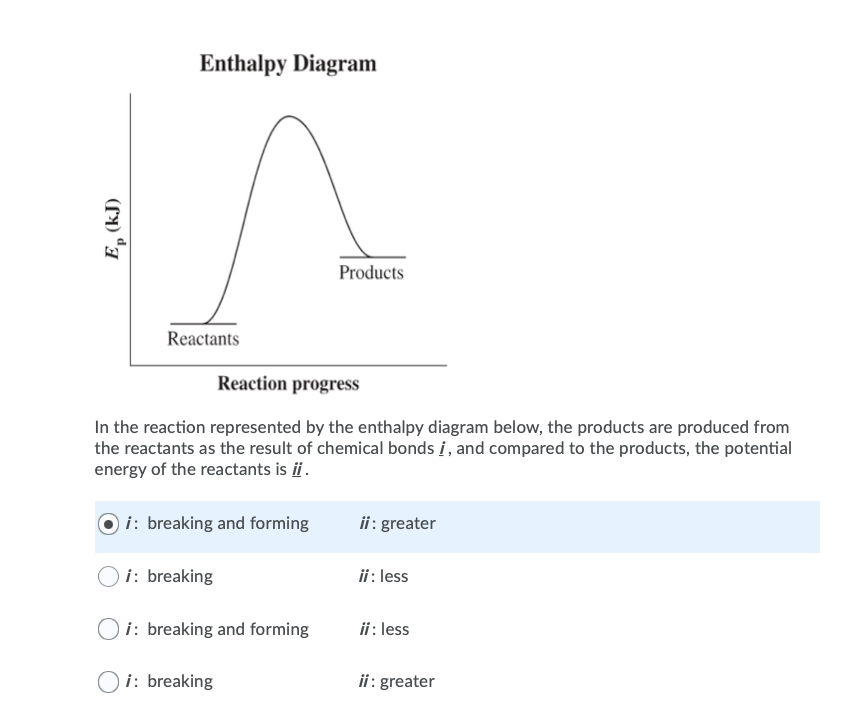
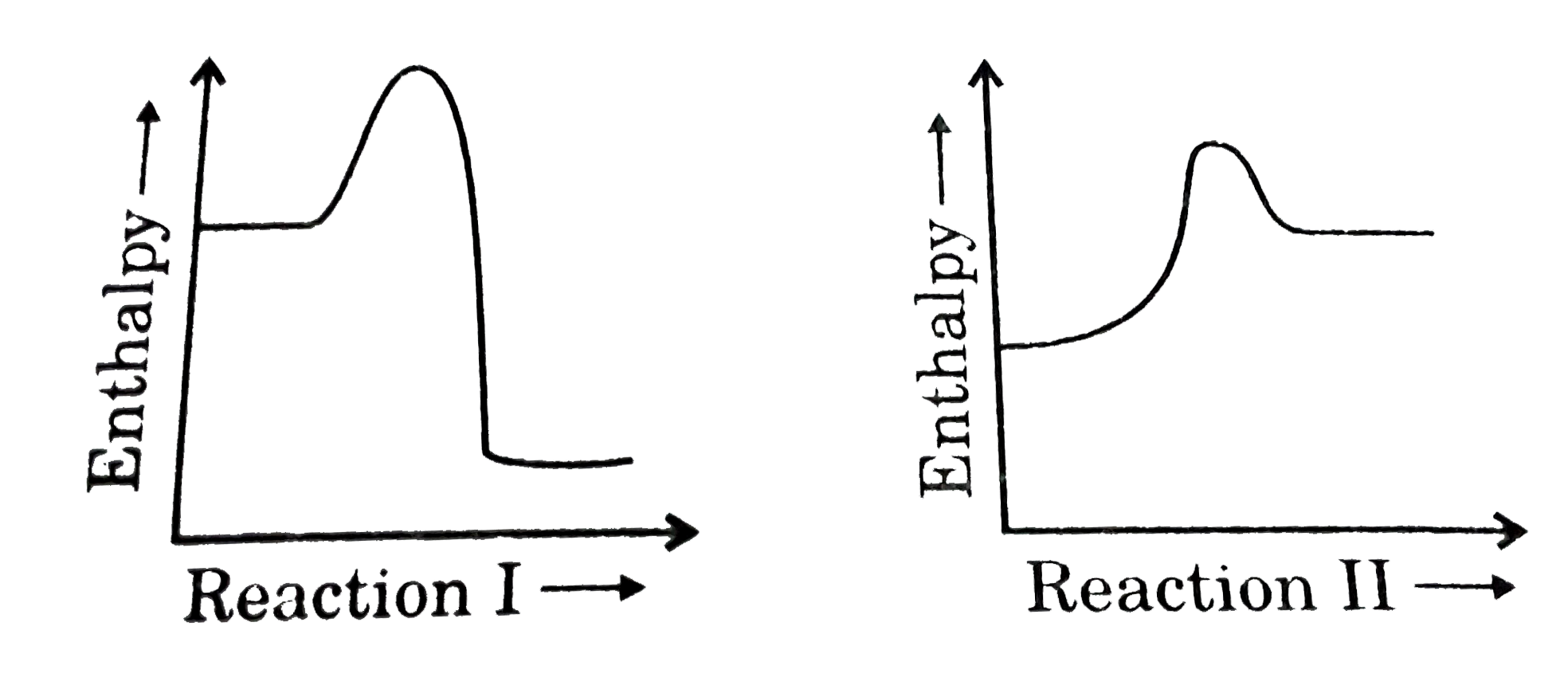
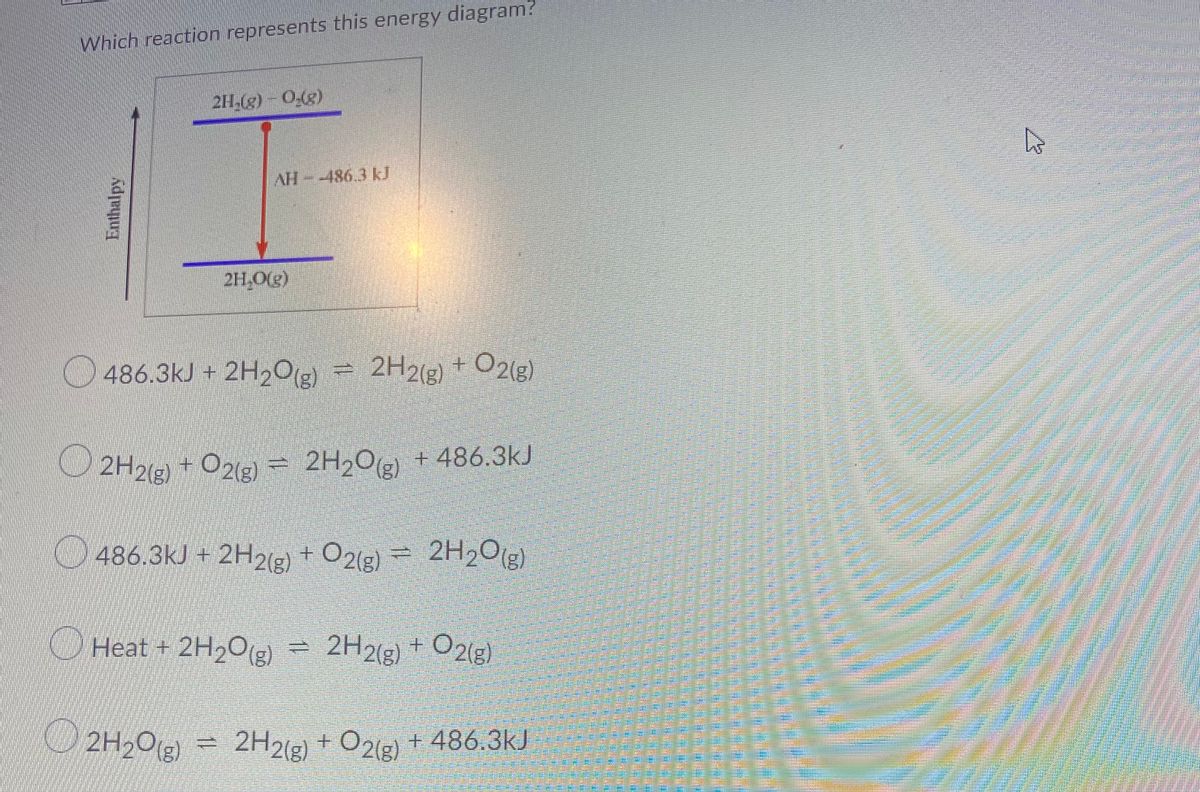
39 which of the following enthalpies of reaction would the reaction represented by the diagram have
Chemistry B-Enthalpy of Reaction-Assignment/Quiz ... The overall change in enthalpy of a reaction depends only on the reactants and the products. According to Hess's law, if a series of intermediate reactions are combined, the enthalpy change of the overall reaction is the sum of the enthalpy changes of the intermediate reactions. Consider the following intermediate chemical equations. mc007-1.jpg Enthalpy of a reaction assignment and quiz Flashcards ... Consider the following enthalpy diagram and enthalpies of intermediate and overall chemical reactions. mc025-1.jpg Which of the following statements is true? The overall chemical reaction is exothermic. There are two intermediate reactions in this system. The third intermediate reaction is endothermic. The longest arrow represents the overall ...
ChemTeam: Hess' Law - using standard enthalpies of formation All the above values have units of kJ/mol because these are standard values. All standard enthalpies have the unit kJ/mol. As a brief reminder, here is the chemical reaction for the standard enthalpy of glucose: 6C(s, graphite) + 6H 2 (g) + 3O 2 (g) ---> C 6 H 12 O 6 (s) Each standard enthalpy value is associated with a chemical reaction.

Which of the following enthalpies of reaction would the reaction represented by the diagram have
Enthalpy of Chemical Reactions - Nassau Community College reaction. Consider the following general type of reaction . reactant Æ products . The ∆H is defined as the difference between the enthalpies of products and the reactants. Thus . ∆H = Hproducts - Hreactants . The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or ... Enthalpies for Different Types of Reactions: Enthalpy ... We can calculate the reaction enthalpy by subtracting the sum of enthalpies of all the reactants from that of the products. Mathematically, Δ t H = Sum of enthalpies of the product – Sum of the enthalpies of the reactants. This is a brief outline of the concept of enthalpy. However, our main aim in this chapter is to earn about the ... 5.4: Enthalpy of Reaction - Chemistry LibreTexts To measure the energy changes that occur in chemical reactions, chemists usually use a related thermodynamic quantity called enthalpy ( H) (from the Greek enthalpein, meaning "to warm"). The enthalpy of a system is defined as the sum of its internal energy U plus the product of its pressure P and volume V: (5.4.2) H = U + P V
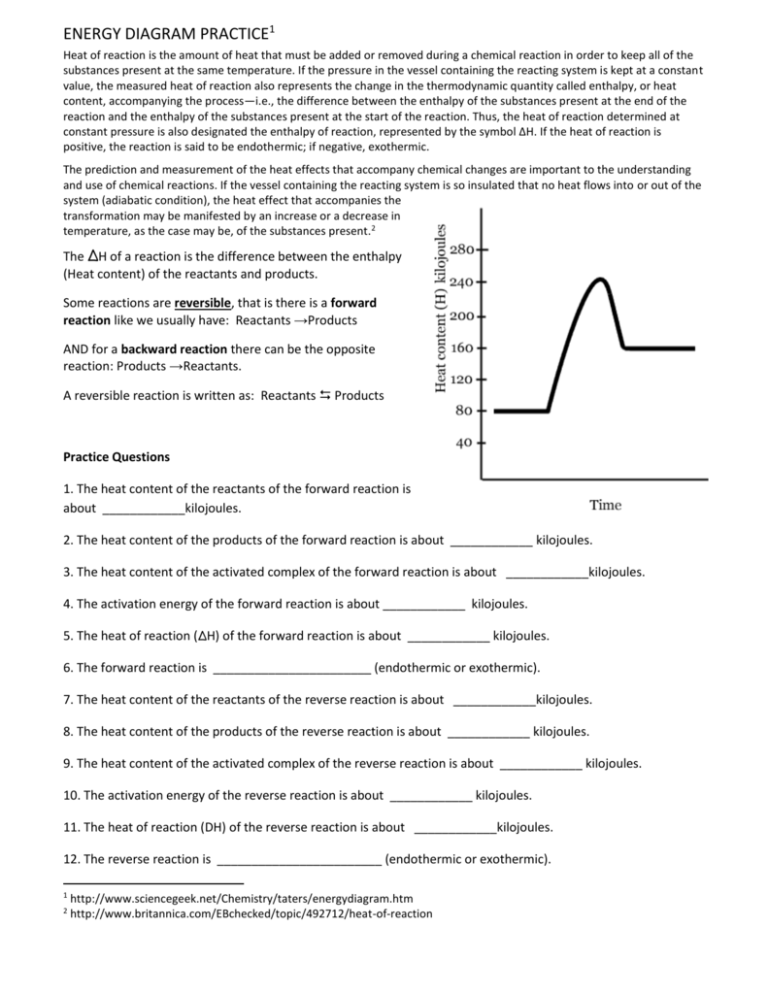
Which of the following enthalpies of reaction would the reaction represented by the diagram have. 5.7: Enthalpy Calculations - Chemistry LibreTexts Enthalpy is a state function which means the energy change between two states is independent of the path. Since equation 1 and 2 add to become equation 3, we can say: ΔH3 = ΔH1 + ΔH2 Enthalpies for Different Types of Reactions: Enthalpy ... For any such reaction, we represent the enthalpy change as Δ r H. We term it as the reaction enthalpy. We can calculate the reaction enthalpy by subtracting the sum of enthalpies of all the reactants from that of the products. Mathematically, Δ t H = Sum of enthalpies of the product - Sum of the enthalpies of the reactants Consider the following enthalpy diagram and enthalpies of ... The enthalpy of the third intermediate reaction is represented as . This reaction shows the conversion of highly energetic unstable intermediate into products. Since unstable intermediate is to be converted into stable products, this change is accompanied by the release of energy. Chapter 10 Energy Changes in Chemical Reactions Flashcards ... heat; work. The change in enthalpy (ΔH) is equal to the change in heat (q) when the system is at constant _____. pressure. True or false: A chemical reaction is observed to take place in a flask. When the reaction is complete, the flask feels colder than it was before the reaction took place. The reaction was exothermic.
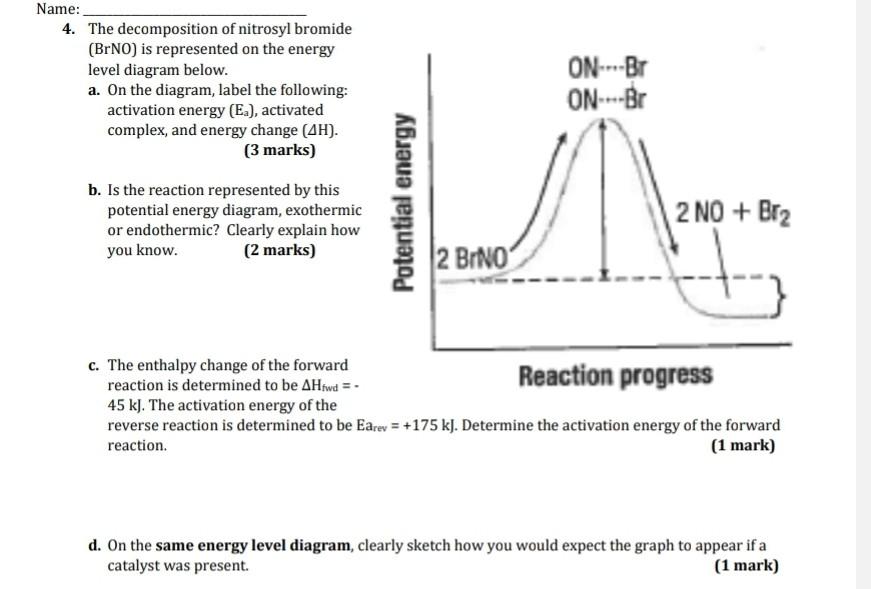
Letter___shows the activation energy for the reaction 4. The activated complex is represented by letter C Explanation: 1. Letter __ shows the overall enthalpy of reaction. The overall enthalpy of reaction is equal to the change of enthalpy of the substances: Enthalpy of reaction = ∑ enthalpy of the products - ∑enthalply of the reactants. The products are shown of the right side of the diagram. 3 Ways to Calculate the Enthalpy of a Chemical Reaction ... Enthalpies of formation are set ∆H values that represent the enthalpy changes from reactions used to create given chemicals. If you know the enthalpies of formation required to create products and reactants in an equation, you can add them up to estimate the enthalpy much as you would with bond energies as described above. Calculating Enthalpy Changes Using Hess's Law Answer: The change in enthalpy for the reaction is -1075.0 kJ/mol. Facts About Hess's Law Hess's Law takes its name from Russian chemist and physician Germain Hess. Hess investigated thermochemistry and published his law of thermochemistry in 1840. Enthalpy of Chemical Reactions - Nassau Community College reaction. Consider the following general type of reaction . reactant Æ products . The ∆H is defined as the difference between the enthalpies of products and the reactants. Thus . ∆H = Hproducts - Hreactants . The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or ...
which of the following enthalpies of reaction would the ... Answer 3 AlWaleed In chemical reactions, the internal energy represents the total energy of the system and is often called Enthalpy. The x-axis represents the reaction progress. Chemical reactions proceed (or are read) from left to right. Enthalpy of Reaction Assignment and Quiz Flashcards | Quizlet Consider the equations below. (1) Ca (s) + CO2 (g) + 1 2 O2 (g) → CaCO3 (s) (2) 2Ca (s)+O2 (g) → 2CaO (s) How should you manipulate these equations so that they produce the equation below when added? Check all that apply. CaO (s) + CO2 (g) → CaCO3 (s) reverse the direction of equation (2) multiply equation (2) by 1/2 Chemistry 4.09 Quiz: Writing Thermochemical Equations ... In which of the following thermochemical equations would the ΔH be considered a heat of solution? NH4Cl (s) → NH4 (aq) + Cl- (aq), ΔH = +14.8 kJ/mol Which of the following enthalpies of reaction would the reaction represented by the diagram have? 8.3: Enthalpy and Hess' Law - Chemistry LibreTexts The reaction uses 8 mol KClO 3, and the conversion factor is − 43.7kJ 0.0587molKClO3, so we have ΔH = 8mol × − 43.7kJ 0.0587molKClO3 = − 5960 kJ. The enthalpy change for this reaction is −5960 kJ, and the thermochemical equation is: C 12H 22O 11 + 8KClO 3 12CO 2 + 11H 2O + 8KCl ΔH = − 5960 kJ. Exercise 8.3.2.
5.4: Enthalpy of Reaction - Chemistry LibreTexts 08.11.2021 · The enthalpy of a system is defined as the sum of its internal energy U plus the product of its pressure P and volume V: (5.4.2) H = U + P V. Because internal energy, pressure, and volume are all state functions, enthalpy is also a state function. So we can define a change in enthalpy ( Δ H) accordingly.
Standard Enthalpy of Formation and Reaction | Boundless ... The following equation can be used to calculate the standard enthalpy of reaction: ΔH⊖ rxn =∑ΔH⊖ f {products}−∑ΔH⊖ f {reactants} Δ H r x n ⊖ = ∑ Δ H f ⊖ { products } − ∑ Δ H f ⊖ { reactants }. The enthalpy of reaction is calculated under standard conditions (STP). Key Terms
Enthalpy of a reaction assignment and quiz Flashcards ...
Chemistry 4.09 Quiz: Writing Thermochemical Equations ...
Enthalpy - Chemistry 2e Determine the enthalpy change per mole of zinc reacting for the reaction: Answer: Δ H = −153 kJ. Be sure to take both stoichiometry and limiting reactants into account when determining the Δ H for a chemical reaction. Writing Thermochemical Equations A gummy bear contains 2.67 g sucrose, C 12 H 22 O 11.
Chem chapter 10 Practice Flashcards | Quizlet The general reaction scheme A + BC → AC + B represents a ______ reaction, while the general reaction scheme X → Y + Z represents a ______ reaction. A 2.26-M solution of KOH is prepared. Calculate the moles and mass of solute present in a 15.2-mL sample of this solution. The molar mass of KOH is 56.11 g/mol.
PDF UNIT 1 THERMOCHEMISTRY - Nova Scotia Department of Education The symbol for enthalpy is H. Many chemical reactions take place in an open beaker under constant pressure of the gases in the room. The internal energy or enthalpy of a reactant or product can not be measured, but the change in enthalpy can be measured. The symbol for change in enthalpy is ∆H. The relationship is ∆H = H products - H reactants
CHM1045 Enthalpy Lecture - Department of Chemistry ... Standard Enthalpy of Reaction. The standard enthalpy of reaction (ΔH o Rxn) is the enthalpy of a reaction carried out at 1 atm. We have already learned one process by which we can calculate the Enthalpy of Reaction in Calorimetry. There are two other methods we will learn now: 1) Heat of Reaction from Standard Heats of Formation
Enthalpies of Reactions - Chemistry LibreTexts 15.08.2020 · Enthalpies of Reactions. Since bond energies are given, we use the monoatomic gases as the reference level in this calculation. The energy level diagram shown below illustrates the principle of conservation of energy, and you are expected to have the skill to draw such a diagram. This diagram is very similar to those of the Born-Haber cycle ...
PDF 6 g What is the standard enthalphy change ΔH Which of the following expressions is equivalent to ΔHo for the reaction represented above? A x + y B x - y C x + 2y D 5. How much heat is released or absorbed when 0.050 mol of Cl2(g) is formed from KCl(s)? 87.4 kJ is released 43.7 kJ is released 43.7 kJ is absorbed 87.4 kJ is absorbed 6.
Thermochemistry Practice Test 2020 Flashcards - Quizlet
PDF Unit 6 - ThermoChemistry is represented by the equation above. a) Use the enthalpies of formation in the table below to calculate the value of ∆H 1 for the reaction. a)e response gives the both the following set up and calculation: Th ∆H reaction = ∑∆H f ° products - ∑∆H f ° reactants ∆H reaction = ( (2 x -394) + (2 x -286) ) - ( 52 ) = - 1412 kJ mol rxn-1
PDF AP Chemistry Student Sample 2, 2017 - College Board The response earned 1 point in part (b) for subtracting the enthalpies of bonds formed from the enthalpies of bonds broken and 1 point for the correct calculation of ∆ H In part (c) 1 point was earned for the explanation that the entropy change would be close to zero because 1 mole of gas is converted into 1 mole of a different gas.
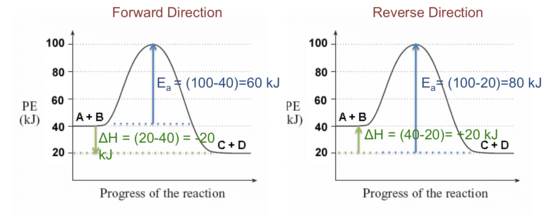
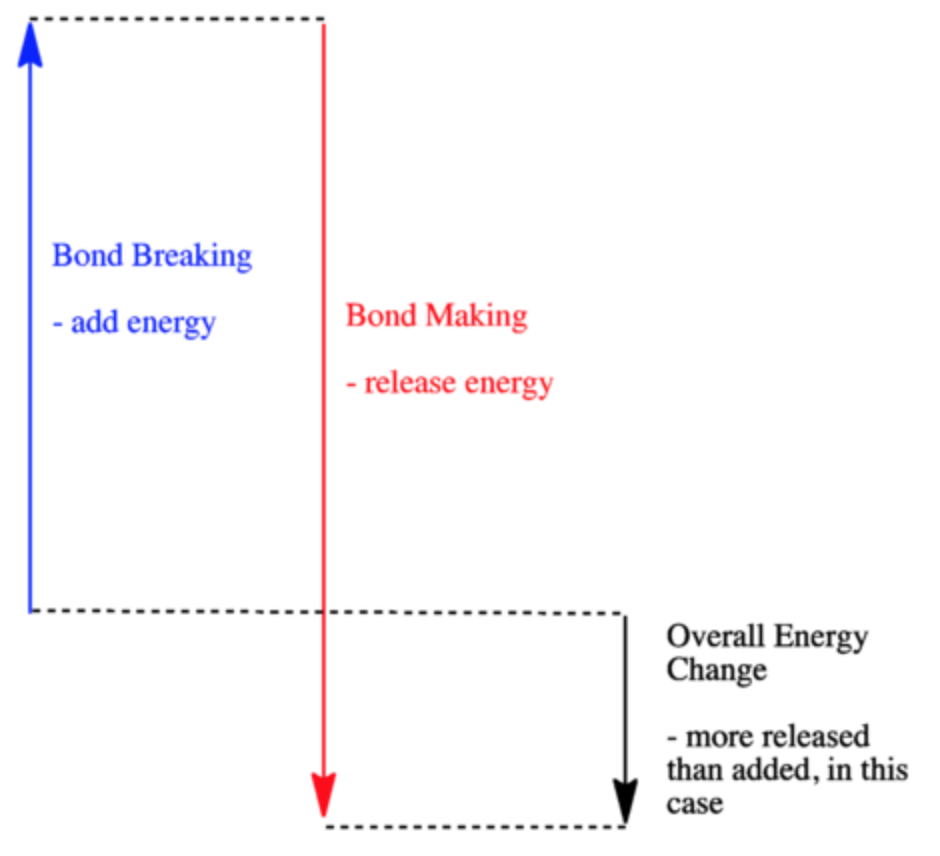
HW Solutions #9 - Chemistry LibreTexts A) The reaction is endothermic B) Reactants are located on the flat portion to the left of the peak. Products are located on the flat portion to the right of the peak. The activated complex is located at the peak of the reaction diagram. C) The enthalpy change is +50 kJ D) The activation energy is 200 kJ E) 50 kJ is absorbed in this reaction
AP Chemistry: Midterm Review Flashcards - Quizlet An estimate for the enthalpy change in a reaction can be made by taking the sum of the bond energies of all the bonds broken and then subtracting the sum of the bond energies of all the bonds formed. In this reaction, a total of 2 moles of C≡O bonds and 1 mole of O=O bonds are broken, while a total of 4 moles of C=O bonds are formed.
Enthalpy and Chemical Reactions - Introductory Chemistry ... The enthalpy change for a reaction is typically written after a balanced chemical equation and on the same line. For example, when two moles of hydrogen react with one mole of oxygen to make two moles of water, the characteristic enthalpy change is 570 kJ. We write the equation as: A chemical equation that includes an enthalpy change is called a
PDF 3.14 Bond Enthalpy - chemrevise 3 C C O H H H H H H O O O C O O H H + 3 2 + 3 1 C-C, 5C-H 1C-O 1O-H and 3 O=O bonds are broken 4 C=O and 6 O-H bonds are made Enthalpies of combustion in a homologous series When comparing the heats of combustion for successive members of a homologous series such as alkanes or alcohols there is a constant rise in the size of the heats of combustion as the number of ...
5.4: Enthalpy of Reaction - Chemistry LibreTexts To measure the energy changes that occur in chemical reactions, chemists usually use a related thermodynamic quantity called enthalpy ( H) (from the Greek enthalpein, meaning "to warm"). The enthalpy of a system is defined as the sum of its internal energy U plus the product of its pressure P and volume V: (5.4.2) H = U + P V
Enthalpies for Different Types of Reactions: Enthalpy ... We can calculate the reaction enthalpy by subtracting the sum of enthalpies of all the reactants from that of the products. Mathematically, Δ t H = Sum of enthalpies of the product – Sum of the enthalpies of the reactants. This is a brief outline of the concept of enthalpy. However, our main aim in this chapter is to earn about the ...
Enthalpy of Chemical Reactions - Nassau Community College reaction. Consider the following general type of reaction . reactant Æ products . The ∆H is defined as the difference between the enthalpies of products and the reactants. Thus . ∆H = Hproducts - Hreactants . The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or ...














0 Response to "39 which of the following enthalpies of reaction would the reaction represented by the diagram have"
Post a Comment