38 potential energy diagram endothermic
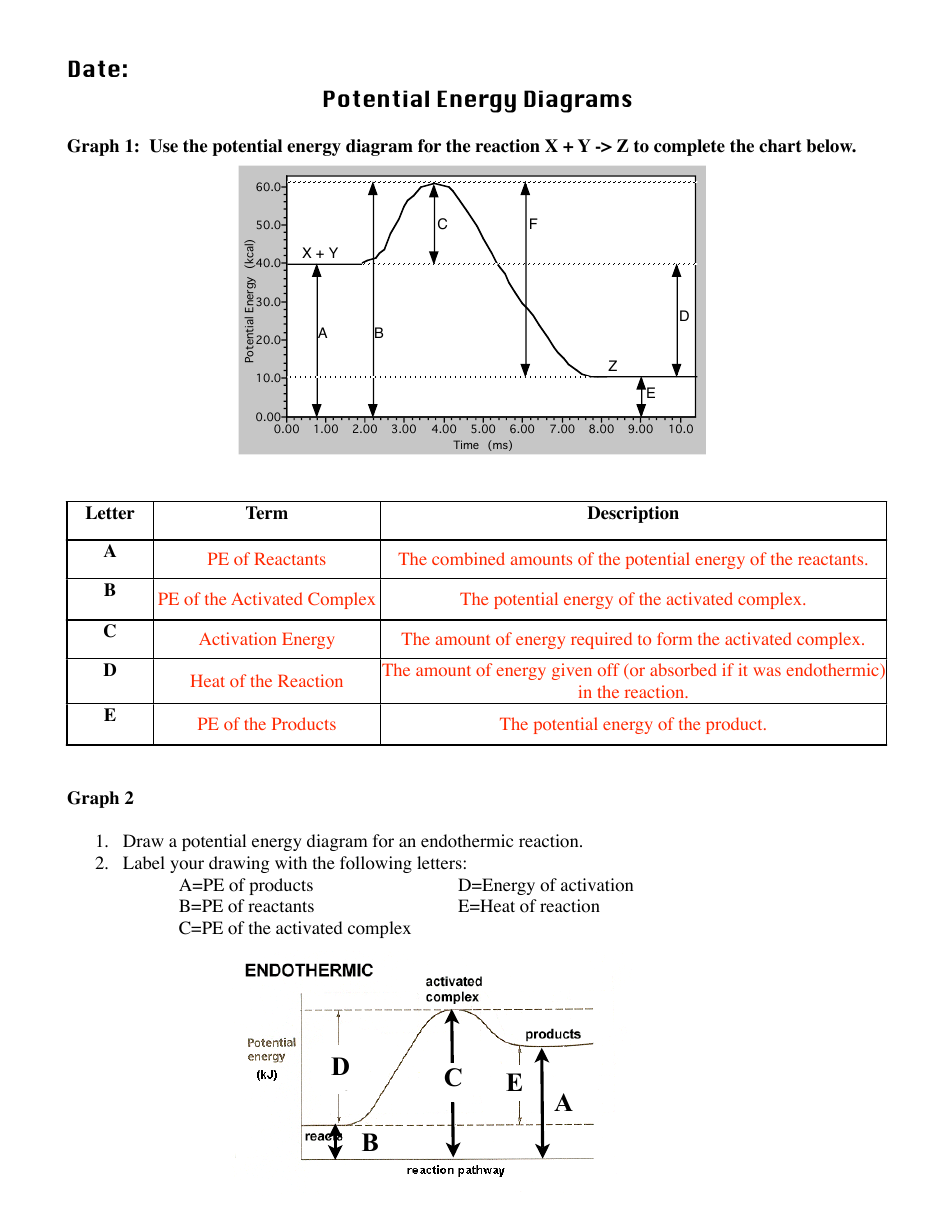
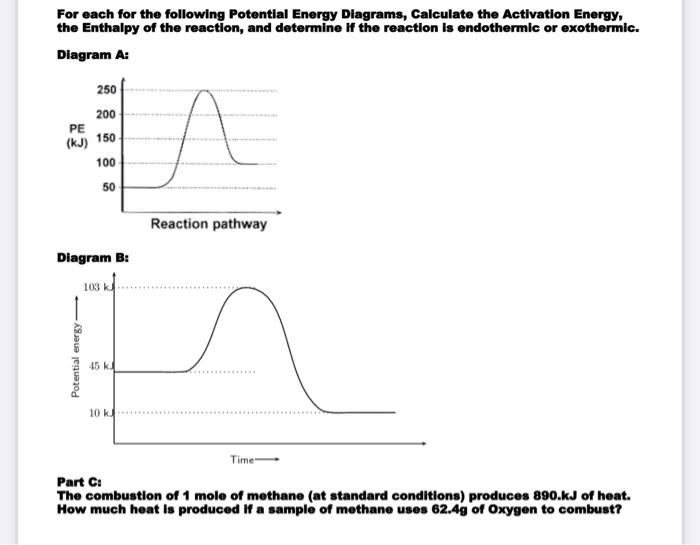
Potential Energy Diagram Practice Endothermic and ... Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse activation energy, answer four conceptual multiple choice questions, and then determine if 9 ... Potential Energy Diagram Endothermic and Exothermic ... Potential Energy Diagram Endothermic and Exothermic A popular example of an endothermic chemical reaction is photosynthesis. Determine the heat of reaction DH for this reaction. The activation energy in an exothermic reaction is energy at the top of the curve minus the energy of the reactants. Endothermic versus exothermic comparison chart.
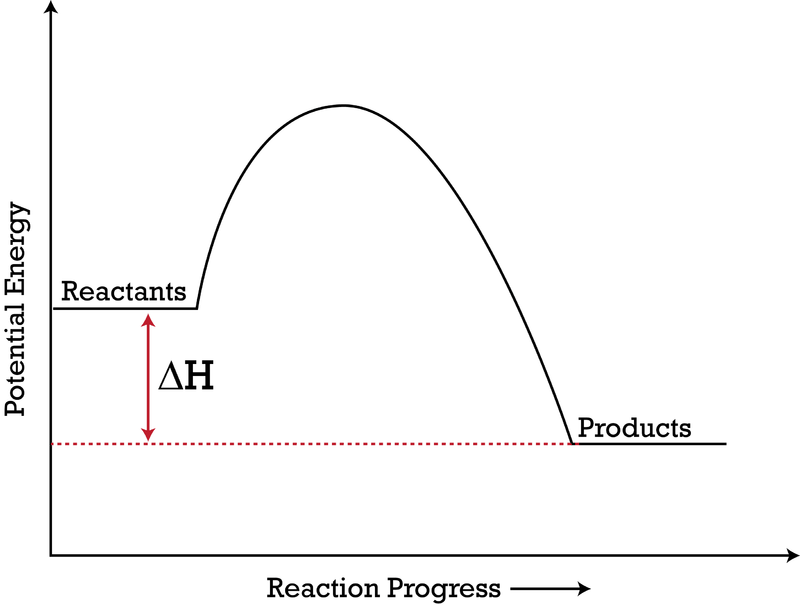
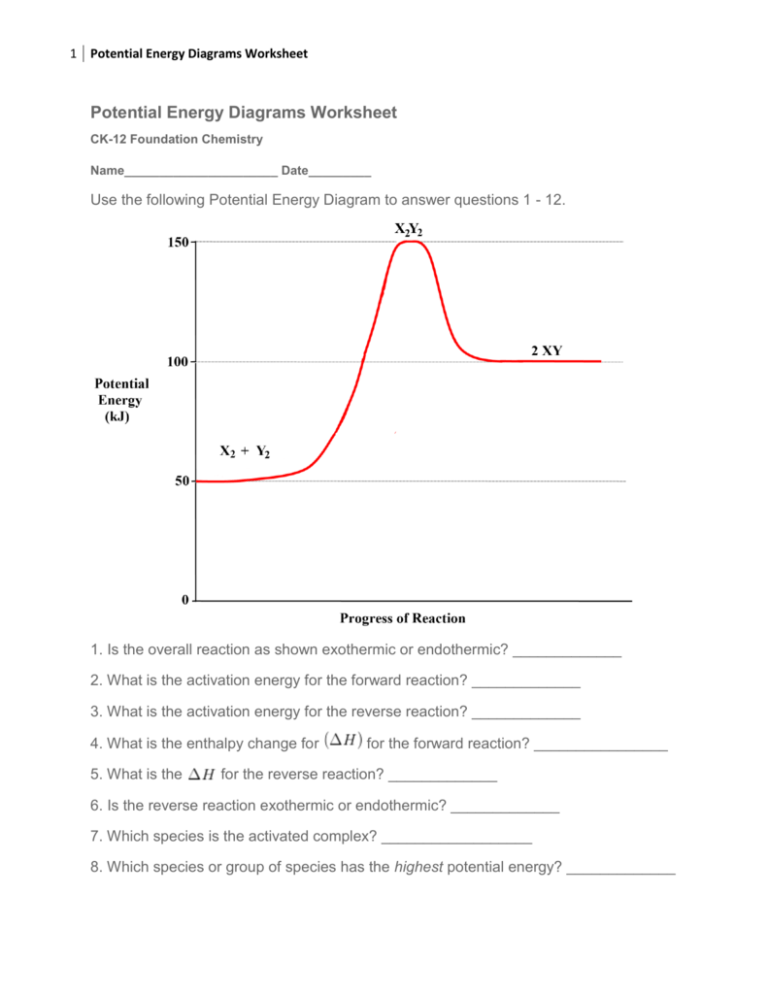
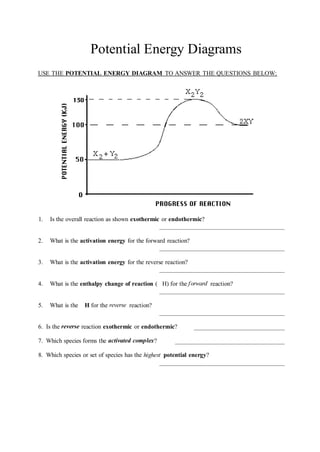
18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction.
Potential energy diagram endothermic
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... ⚗️How does the potential-energy diagram for a reaction ... How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? An endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products. What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
Potential energy diagram endothermic. Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. 6.6: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction. Which statement is true about the potential energy diagram ... Answer: Products have less potential energy than reactants. In an endothermic reaction, the reaction mixture will absorb heat from the surroundings. Therefore, the products will have higher energy than the reactants and hence, ΔH will be positive. In an exothermic reaction, the reaction mixture will release heat to the surroundings. PDF Potential Energy Diagrams THE DIAGRAMS GRAPHICALLY COMPARE REACTANT ENERGY TO PRODUCT ENERGY AS REACTION OCCURS IF REACTANTS HAVE MORE ENERGY THEN PRODUCTS, THE REACTION IS EXOTHERMIC (loss of energy) IF REACTANTS HAVE LESS ENERGY THEN PRODUCTS, THE REACTION IS ENDOTHERMIC (gain of energy) ENDOTHERMIC OR EXOTHERMIC? Note the REACTANTS and PRODUCTS are labeled.
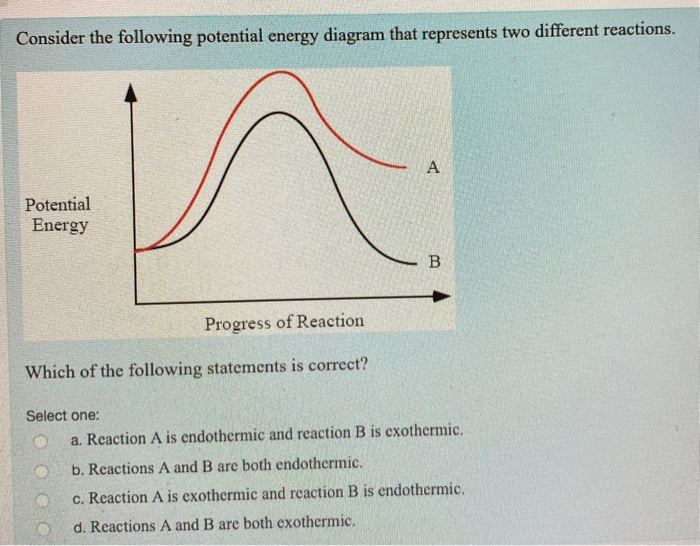
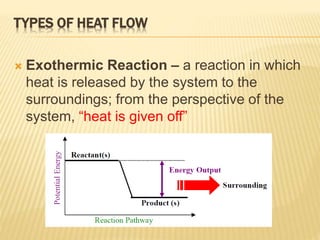
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ... Write a two to four sentence conclusion statement ... If the potential energy diagram has the product(s) having a higher potential energy than the reactant(s), then the reaction is an endothermic reaction (this is why the reaction absorbs heat from the surroundings to try to make up for the lack of potential energy of the reactants) and the sign on the enthalpy change is visibly positive since H ... en.wikipedia.org › wiki › Lattice_energyLattice energy - Wikipedia The concept of lattice energy was originally applied to the formation of compounds with structures like rocksalt and sphalerite , where the ions occupy high-symmetry crystal lattice sites. In the case of NaCl, lattice energy is the energy change of the reaction Na + (g) + Cl − (g) → NaCl (s) which amounts to -786 kJ/mol. Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
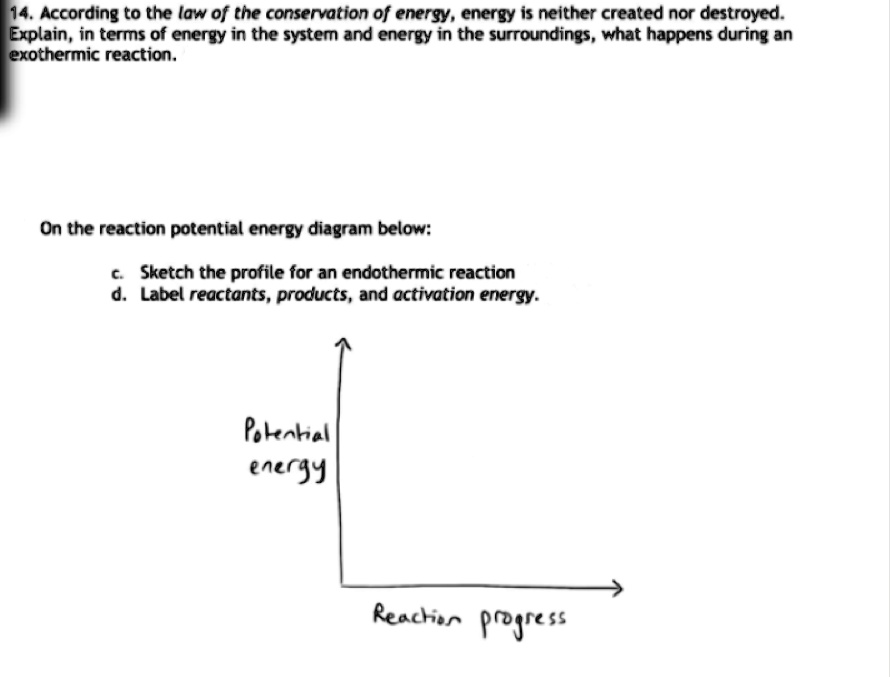
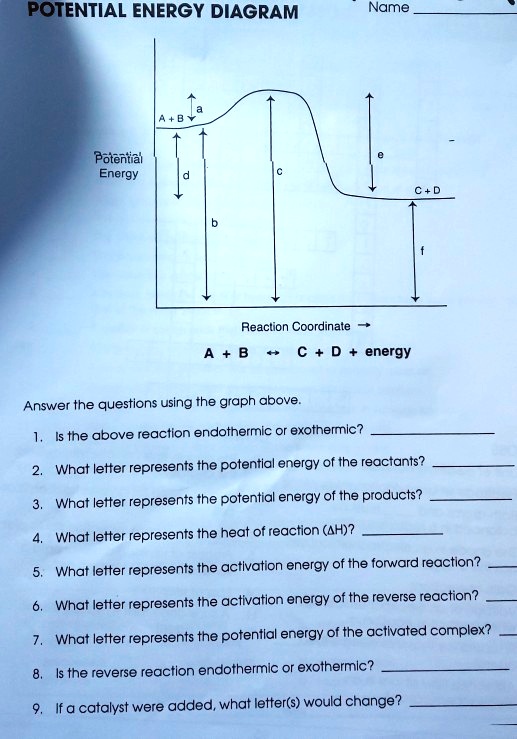
Solved If we draw a potential energy diagram for an ... Transcribed image text: If we draw a potential energy diagram for an endothermic reaction, the potential energy of reactant is less than potential energy of product equal to the potential energy of the product more than potential energy of product equal to activation energy of reverse reaction equal to activation energy of forward reaction 07_01_journal.docx - Chemistry Journal 7.1 Endothermic and ... Chemistry Journal 7.1 Endothermic and Exothermic Driving Question: How are energy transfers during chemical reactions explained and represented by potential diagrams? Key Ideas and Terms Notes FQ: What is the relationship between heat, temperature, and thermal energy within a system? What is thermochemistry? a branch of chemistry concerned with the quantities of heat released or absorbed ... PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ... 07_01_journal.doc - Chemistry Journal 7.1 Endothermic and ... A potential energy diagram tracks the potential energy of a system over the course of a reaction or process. Notice that the y-axis shows a measure of potential energy, usually in the unit kilojoules, and the x-axis shows the progress of a reaction. A potential energy diagram is not like an (x, y) ordered pair plot you may be used to in math.
Which statement describes the potential energy diagram of ... Endothermic reactions are defined as the reactions in which energy is absorbed by the system. The potential energy of the products is greater than the potential energy of the reactants. The total enthalpy change of the reaction, comes out to be positive. for endothermic reactions
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
In a potential energy diagram for an endothermic reaction ... Here is a potential energy diagram for an endothermic reaction. (Adapted from 1.bp.blogspot.com) 1. False ΔrH = H products − H reactants > 0 2. True Ea = Eactivated complex − Ereactants > 0 3. True The red arrow in the diagram represents Ea, reverse. Ea, reverse < Ea, forward because Eproducts > Ereactants 4. False Eproducts > Ereactants
Representing endothermic and exothermic processes using ... A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential energy than the final state, the process is endothermic.
Endothermic and Exothermic Activity.pdf - Endothermic and ... Endothermic and Exothermic Activity For this assignment, you will create your own potential energy diagrams for each of the three chemical reactions. Then you will analyze the data and your diagrams for each reaction. Generic Reactions Reactants Products Transition State Synthesis A + B → AB A + B −15 kJ AB 20 kJ 30 kJ Single Replacement C + AB → CB + A C + AB 65 kJ CB + A 30 kJ 85 kJ ...
How to draw the potential energy diagram for this reaction ... Apr 9, 2018 Since heat is released for C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction.
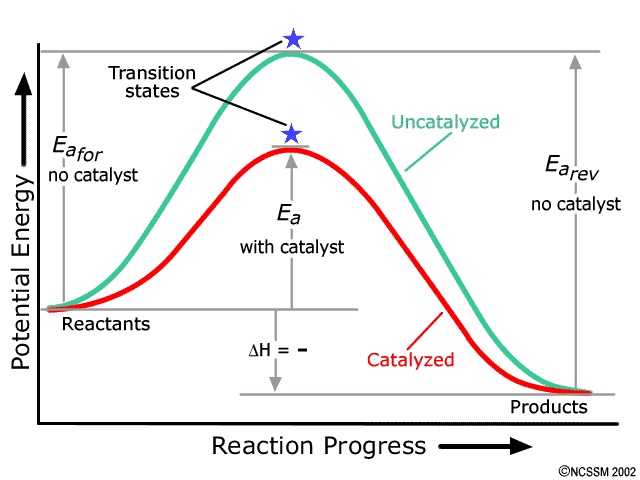
Solved Endothermic reactions 5. In the space below, draw a ... The following diagram is the potential energy diagram for the same exothermic reaction as in question 1. produsenere Supe Stage Reaction coordinate On the diagram, draw a dotted line to show the pathway of a catalyzed reaction. Add the label C to show the interval that represents the activation energy for the catalyzed reaction. 9.
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
⚗️How does the potential-energy diagram for a reaction ... How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? An endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...






















0 Response to "38 potential energy diagram endothermic"
Post a Comment