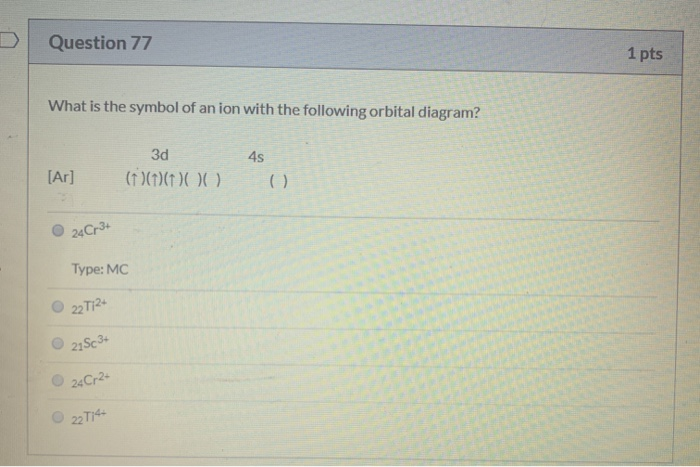
37 cr3+ orbital diagram
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. See the answer See the answer done loading. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+. Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+. Expert Answer.
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ...

Cr3+ orbital diagram
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s. The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. Write orbital diagram for Au+? ↿⇂. ↿⇂. ↿⇂. Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
Cr3+ orbital diagram. Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link. d-orbital subshell leads to higher energies • Each metal orbital will either be stabilized or destabilized depending on the amount of orbital overlap with the ligand. CFT (Octahedron) (d z2, d x2-y2) Orbitals point directly at ligands stronger repulsion higher energy (d xy, d yz, d xz) Orbitals point between ligands Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ... Step 1. 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. Diamagnetic ions contain no unpaired electrons. Paramagnetic ions have unpaired electrons. Determine the orbital diagrams of a. V. 5 + ^ {5+} 5 +. b.
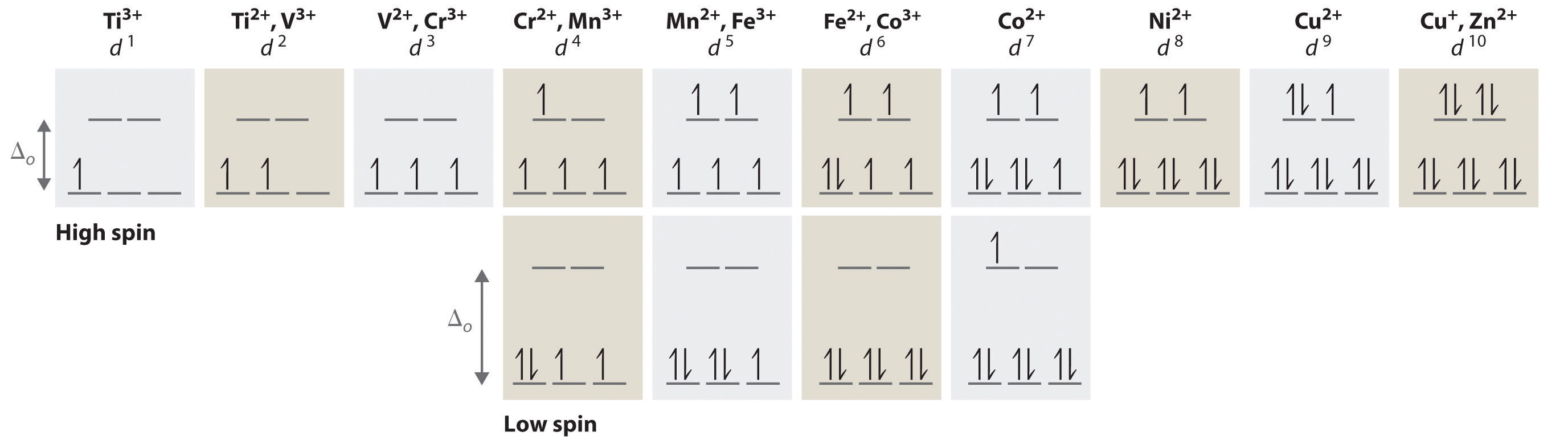
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ... In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ... However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. can be accommodated in the metal d orbitals. • d0 ions d3 ions - V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Mo3+.
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the orbitals are represented by half arrows. electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number...
To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
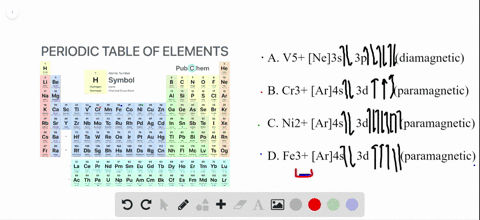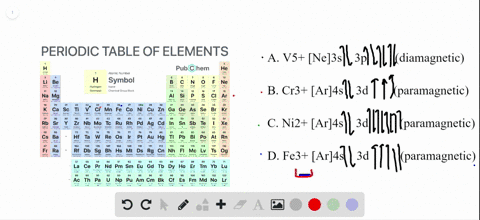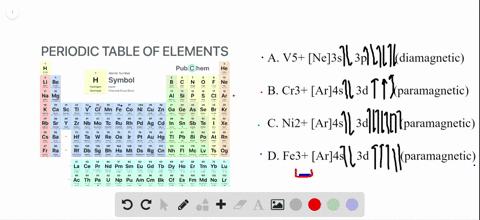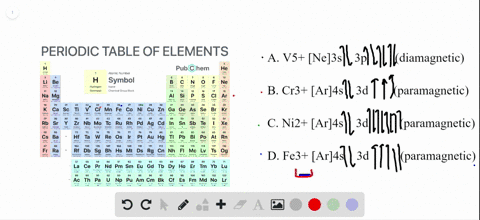
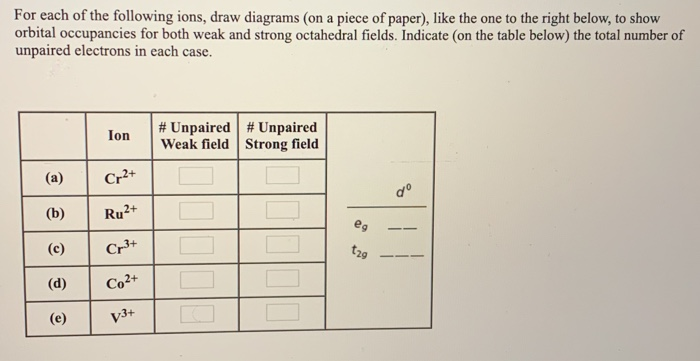
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3
for the molecular orbitals with high d orbital character. metal d orbitals xz yz x2-y2 xy z2 1.125e 2.75e b. For consideration of L as a -acceptor in the axial position, the identical energy level diagram is obtained regardless of whether L is assigned to position 1 or 6. The xz and yz
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
Orbital Diagram For Fe3 Examples: Electron Configurations of Transition Metal Ions hen d-block elements form ions, the 4 s electrons are lost first: Ion Electron Configuration Cr 1s22s22p63s23p6 4s13d 5 Cr3+ 1s22s22p63s23p6 3d3 Ion Electron Configuration Fe 1s22s22p63s23p6 4s23d 6 Fe3+ 1s22s22p63s23p6 3d5 Lost 3 electrons - one from 4 s and two ...
The electronic configuration of Cr having atomic number of 24 is 1s22s22p63s23p64s13d5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.

Solved 41 Use The Periodic Table And Periodic Trends To Identify And Indicate The Symbol And Name The Transition Element Of Period 4 That Has Paramagnetic Cation With Load 2 And The Orbital Full
The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In Br or Ar S or Sn Si or Cl. In Br Sn Si. Choose the ...
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
Answer. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label . the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets ...

Pdf Direct Observation Of Cr3 3d States In Ruby Toward Experimental Mechanistic Evidence Of Metal Chemistry
Sketch the form of the vacant s* orbital of a dihalogen molecule and describe its role in the Lewis acidity of the dihalogens? 3 3 a). b) IF3: X = IF4N(CH3)4 Different possible arrangements of Since the 2σu* antibonding orbital is the LUMO for a X2 molecule, it is the orbital that accepts the pair of electrons from a Lewis base. 17.5
The orbital diagram would show the spin of each electron in the electronic orbital as well as identify the type of orbital being occupied by the electrons. Answer and Explanation: 1.
1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 which is half-filled d-orbital. C r 3 + has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [A r] 3 d 3.
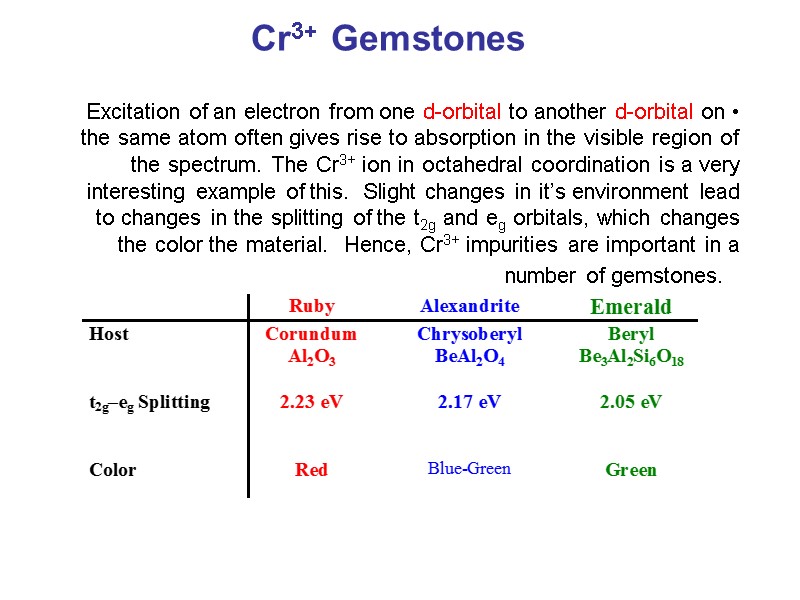
Chem242. Int. Inorg. Chem. Spring, 2007 UMass-Amherst The lowest energy state is designated 4A 2g ("quartet A-two-g") and is the ground state. The "4" tells you the spin multiplicity (# unpaired electrons + 1), while the "A 2g" indicates the symmetry of the electronic state.
3. 3+Using your answer from Question 2, draw a d-orbital splitting diagram for low-spin Co similar to the one given for Cr3+ 3+in the introduction to this experiment. Almost all Co complexes are low-spin, with a minimum number of unpaired electrons. (Refer to your text for further explanation.) 4.
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
The valence electron configuration of Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 instead of 1s2, 2s2, 2p6, 3s2, 3p6, 3d4, 4s2 because one of the electron from the s orbital jumped to the d orbital. By distributing its electrons along the empty orbitals, it becomes more stable. Since the ground state for Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1.
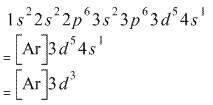
How Is The Electron Configuration Of Cr3 1s2 2s2 2 6 3s2 3p6 3d5 4s1 Ar 3d5 4s1 Ar 3d3 Home Work Help Learn Cbse Forum
Question: Write orbital diagram for Cr3+ This question hasn't been solved yet Ask an expert Ask an expert Ask an expert done loading. Write orbital diagram for Cr 3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. Write orbital diagram for Au+? ↿⇂. ↿⇂. ↿⇂.
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.















0 Response to "37 cr3+ orbital diagram"
Post a Comment