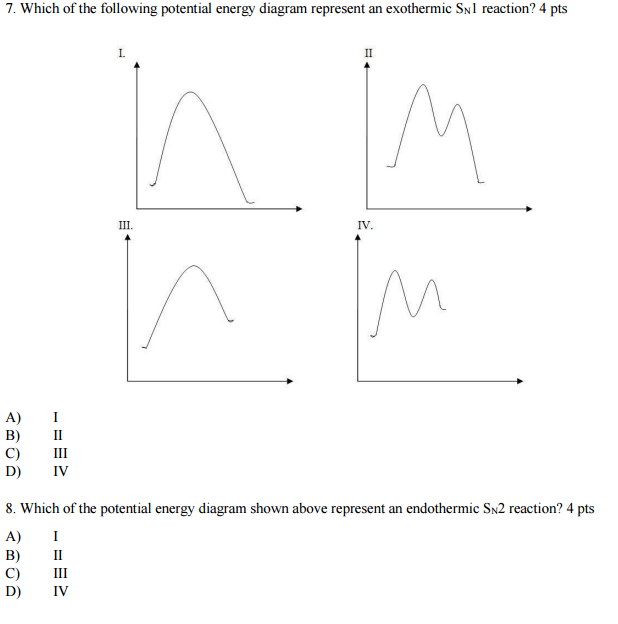
35 potential energy diagram for endothermic reaction
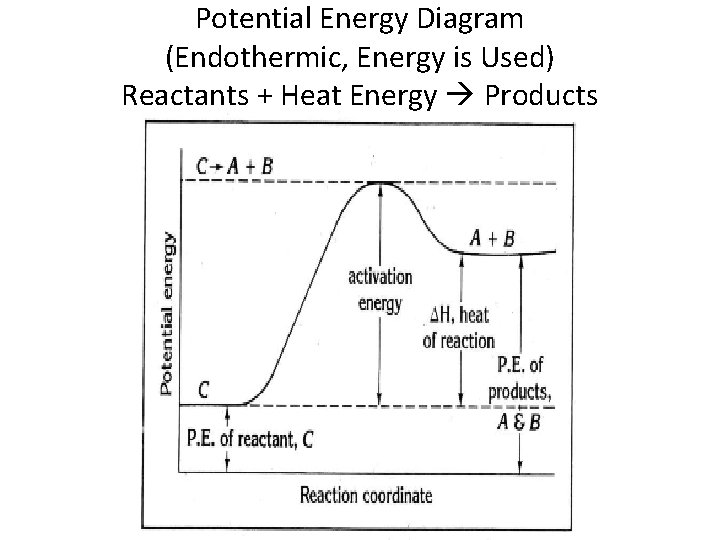
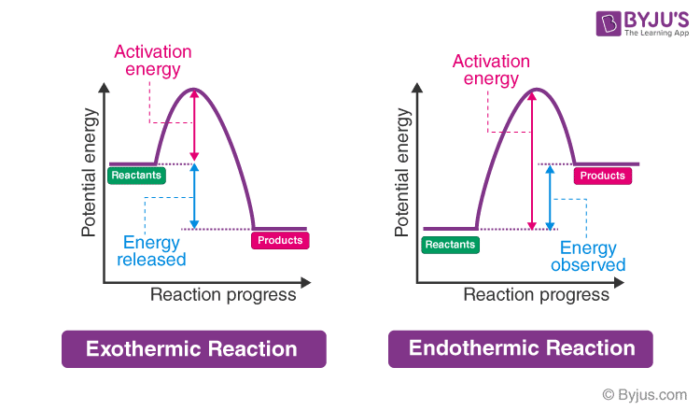
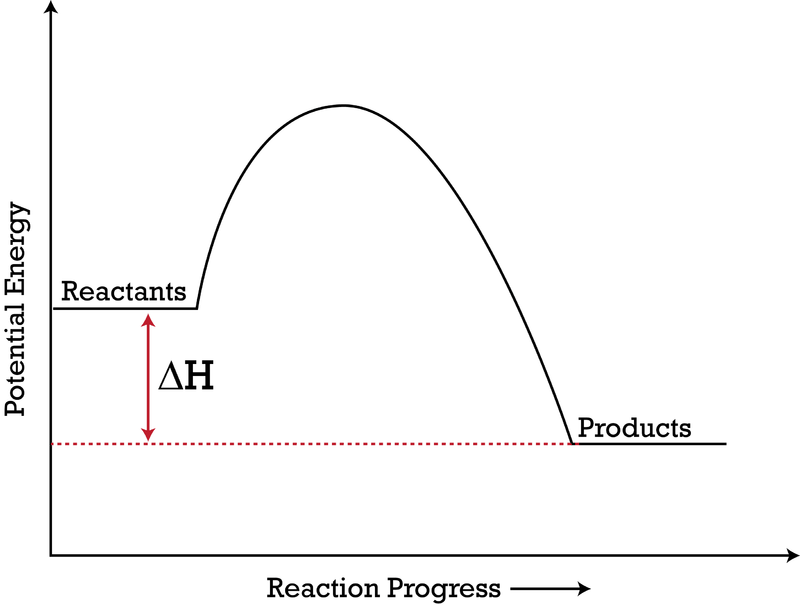
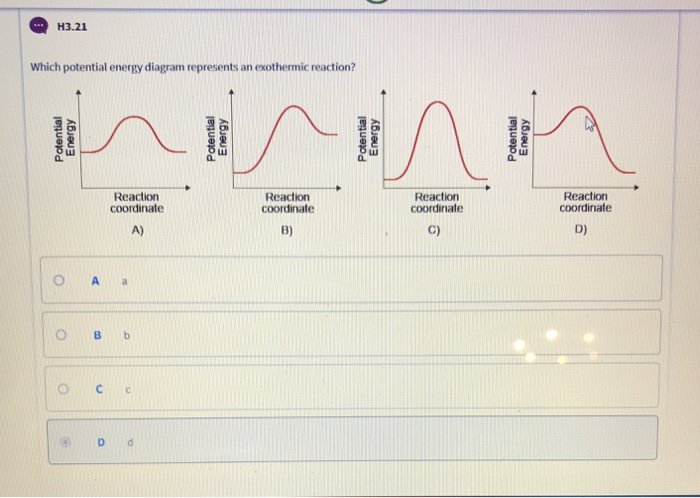
Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time. Does this graph represent an endothermic or an exothermic reaction? Explain your answer. Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. 3.
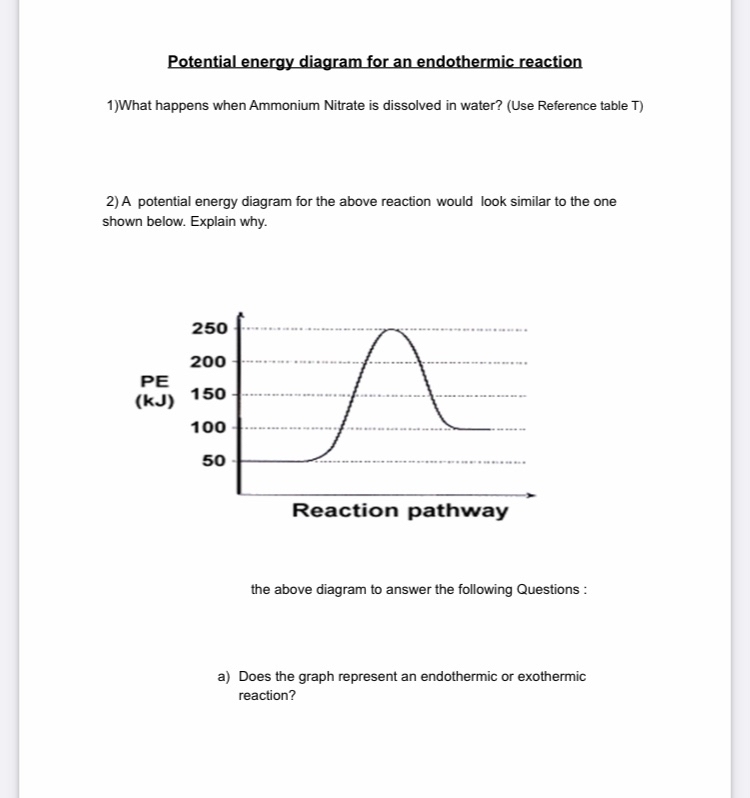
Jul 9, 2019 — A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the ...

Potential energy diagram for endothermic reaction
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... Usually, potential energy diagrams were plotted using reaction pathway/reaction progress in the x-axis and potential energy in the y-axis. For exothermic reactions, the total energy of the products will be leass than the reactants. Since, energy is released during the reaction process. On the other hand, for an endothermic reaction the energy ... 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) E P of the reactants C R b) E P of the products E Z c) ∆H of the reaction B S d) activation energy of the
Potential energy diagram for endothermic reaction. Feb 23, 2012 — Potential energy diagrams show how the potential energy changes during reactions from reactants and products. · Exothermic reactions have a ... In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products. This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\). Potential energy diagrams. Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the ... ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
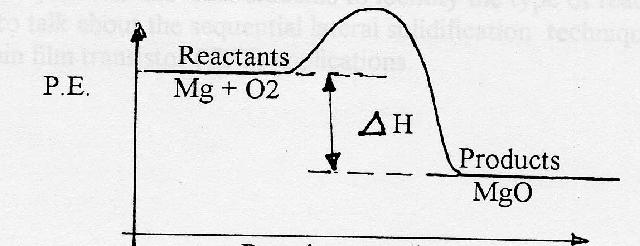
Very exothermic reaction with a small activation energy. Energy level diagram for an endothermic chemical reaction without showing the activation energy. Moderately endothermic reaction with a moderately high activation energy. POTENTIAL ENERGY DIAGRAMS 10. ASSESSMENT - POTENTIAL ENERGY DIAGRAM 1. Is the reaction endothermic or exothermic? 2. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is ... Figure 18.4. 1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and Δ H is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
energy of products is 120 kJ/mol and the activation energy for the forward reaction is 120 kJ/mol. Label the diagram clearly. Is this reaction exothermic or endothermic? Example: Draw an energy level diagram for the reaction described below. (label the axis!) Potential energy of reactants = 350 kJ/mole Activation energy = 100 kJ/mole energy needed for a reaction to happen - this lower activation energy is shown by the dotted red line in the diagram here. Energy level diagrams for endothermic reactions In endothermic reactions the reactants have a less energy than the products . The difference between these two energy levels is the energy absorbed from the surroundings. Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a-f in the diagram represents the potential ... The forward reaction is ____endothermic_____ (endothermic or exothermic). 7. The PE of the reactants of the reverse reaction is about __160___ kilojoules. 8. The PE of the products of the reverse reaction is about ___80__ kilojoules.
The potential enegy of reactants is less… View the full answer Transcribed image text : If we draw a potential energy diagram for an endothermic reaction, the potential energy of reactant is less than potential energy of product equal to the potential energy of the product more than potential energy of product equal to activation energy of ...
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy ...
In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher than the beginning, showing how the system gained energy during the course of the reaction.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
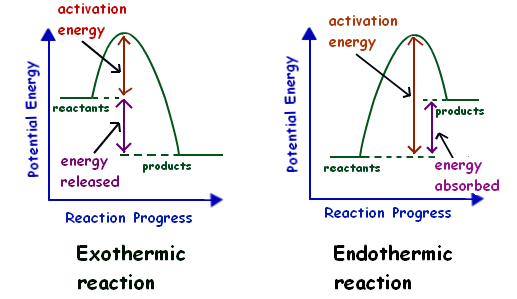
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Energy reactants products exothermic reactions the reactants have more potential energy than the ...
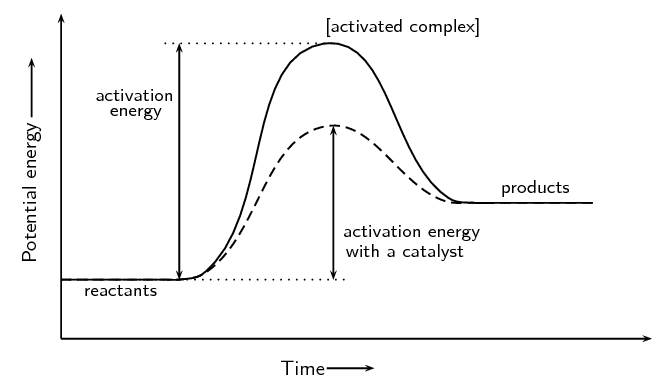
Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ...
Endothermic Reaction Energy Diagram. bbc gcse bitesize energy level diagrams energy level diagrams during a chemical reaction energy is either taken in or given out during exothermic reactions energy is given out this means that the energy of the products will be lower than the energy of the reactants this can be represented using an energy level diagram endothermic vs exothermic reactions ...
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. Is negative when heat is released and the reaction is exothermic. The reactants and products are pretty self explanitory they are the difference between absolute zero and the energy of each on the curve a and g respectively.
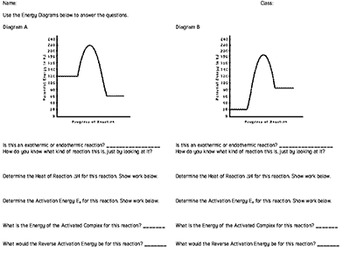
The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of ...
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) E P of the reactants C R b) E P of the products E Z c) ∆H of the reaction B S d) activation energy of the
Usually, potential energy diagrams were plotted using reaction pathway/reaction progress in the x-axis and potential energy in the y-axis. For exothermic reactions, the total energy of the products will be leass than the reactants. Since, energy is released during the reaction process. On the other hand, for an endothermic reaction the energy ...
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....

















0 Response to "35 potential energy diagram for endothermic reaction"
Post a Comment