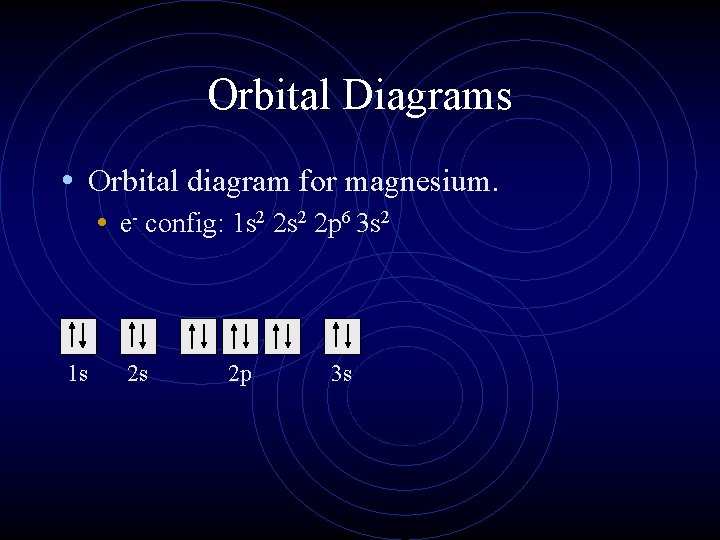
36 orbital diagram for magnesium
What element does the following orbital diagram represent? Mg (magnesium). What element does the the following orbital diagram represent? V (vanadium). Solutions for Chapter 8 Problem 4SAQ. Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4.
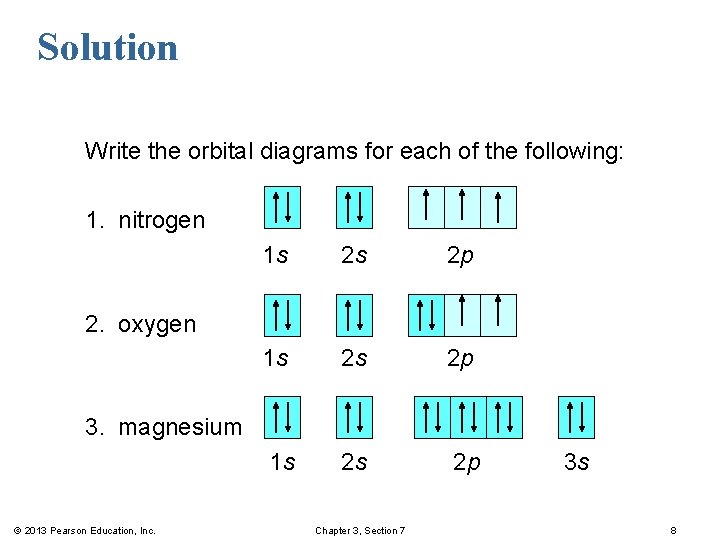
(Recall from Section 5.3B that two electrons in an orbital spin in opposite directions on their axes.) Therefore, if an orbital contains two electrons, its box will contain two arrows, one pointing up and the other down. Using a box diagram, we show the electron configuration of nitrogen as: Notice that the 2p electrons are shown as
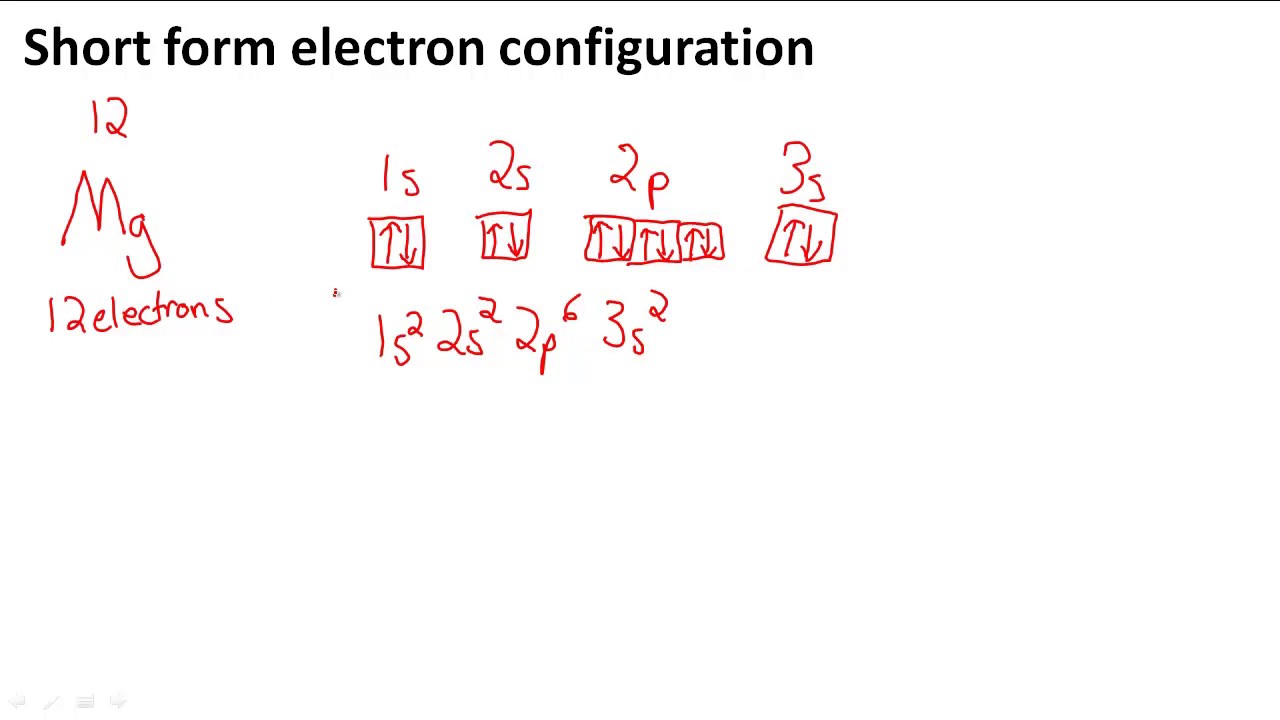
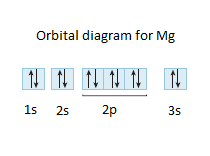
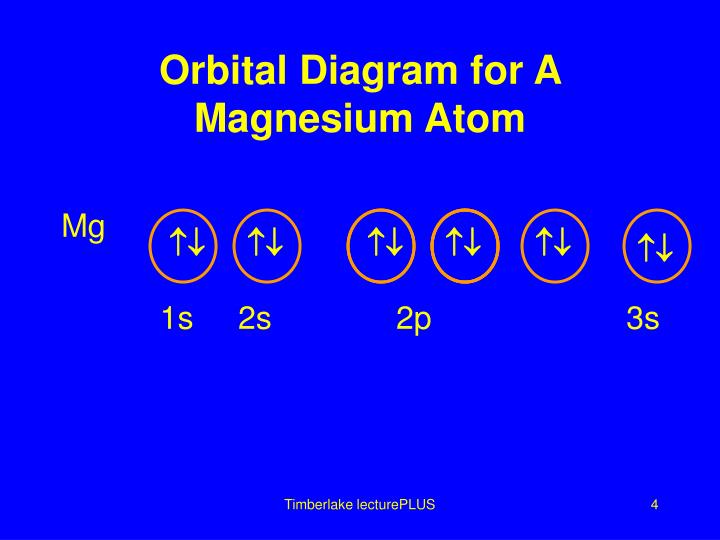
To write the orbital diagram for the Magnesium atom (Mg) first we need to write the electron configuration for just Mg. To do that we need to find the number...

Orbital diagram for magnesium
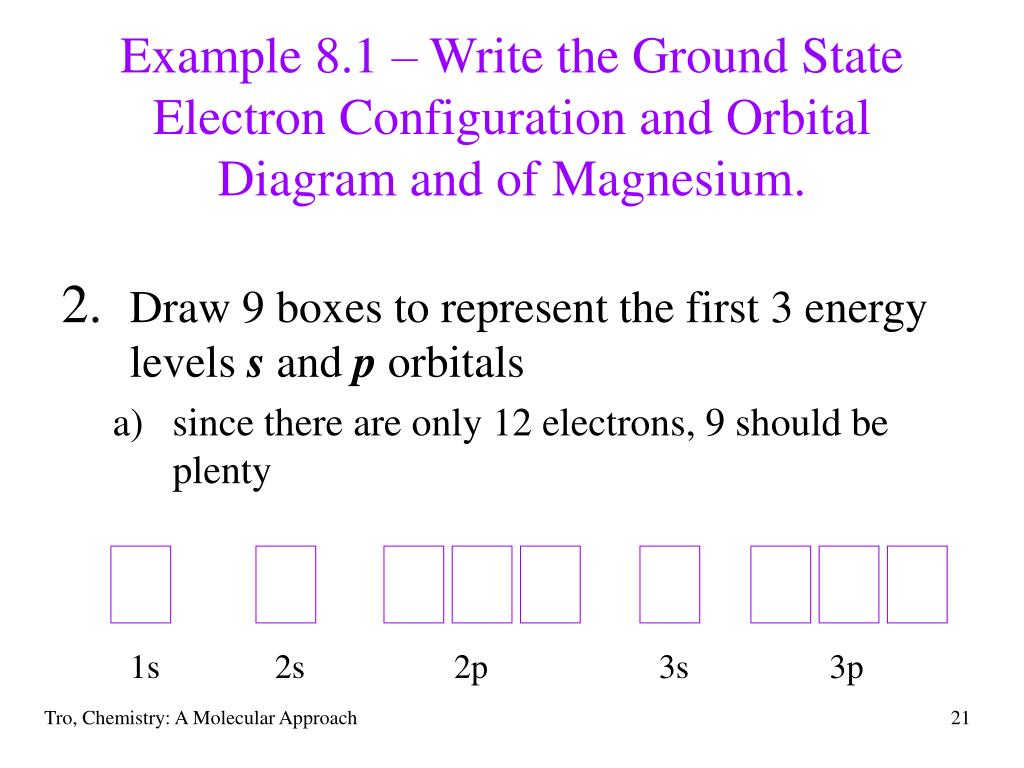
The nex six electrons will go in the 2p orbital. The orbital notation for magnesium is: Magnesium is in the third row and second column of the Periodic Table. what is the orbital notation of magnesium and silicon? Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
Orbital Diagram For Magnesium Summarized by PlexPage. Last Updated: 02 July 2021 * If you want to update the article please login/register. General | Latest Info. Energy levels are given by numerals above boxes, so 1 2 3 etc. The number of core electrons is all electrons that are not at outermost energy level. The number of valence electrons is the number of electrons at the outermost energy ...
The electronic configuration for magnesium is 1s^2 2s^2 2p^6 3s^2. The outermost electron is one in the 3s orbital, which means that n = 3. It also means that l = 0, since s is basically shorthand for the orbital shape of l = 0. m_l can range from -l to +l, but since l = 0, then m_l must also be 0.
Orbital diagram for magnesium.
Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion (Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2.
Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
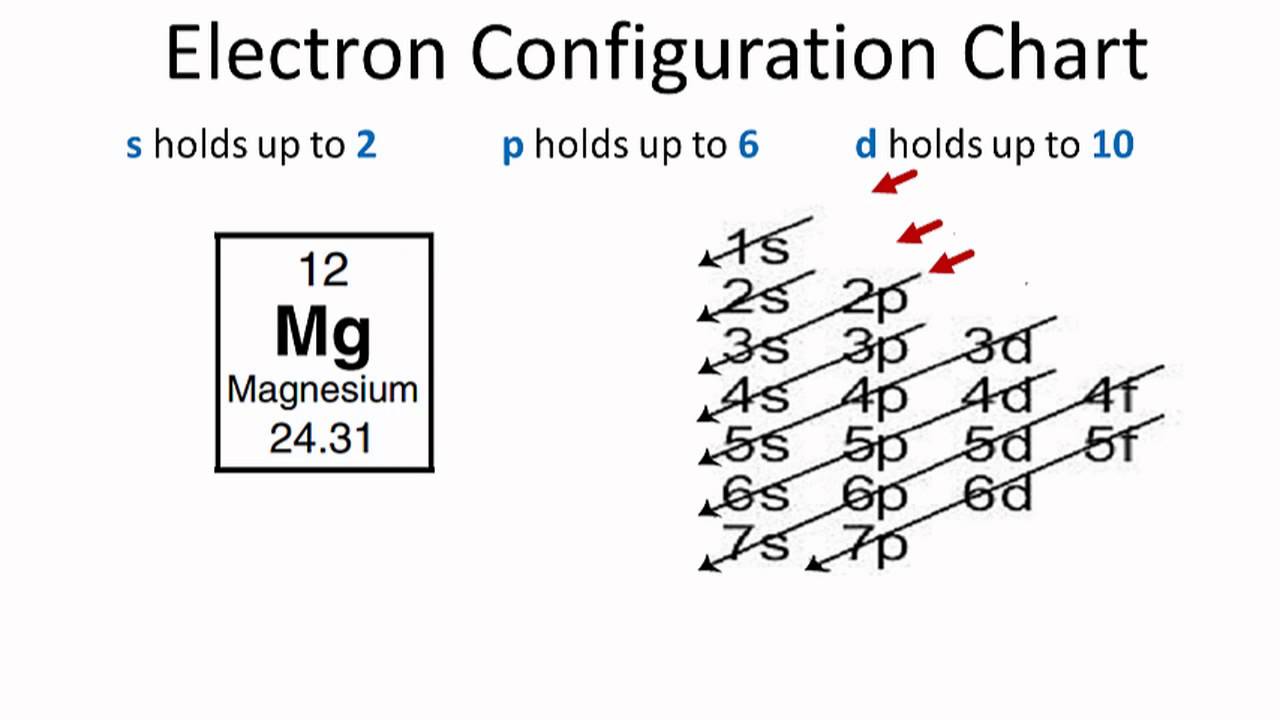
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s.
Write orbital diagrams and electron configurations for the following atoms Nitrogen (N) #e-: 7 Electron Configuration: 1s 2 2s 2 2p 3 Orbital Diagram: Oxygen (O) #e-: 8 Electron Configuration: 1s 2 2s 2 2p 4 Orbital Diagram: Manganese (Mn) #e-: 25 Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 Orbital Diagram: Gallium (Ga) #e-: 31 An orbital diagram, or orbital box …
Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti ...
What is the orbital diagram and electron configuration of magnesium? We’ll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2.
22.9.2021 · The statement that best explains why magnesium and chlorine combine in a 1:2 ratio is; Magnesium has two valence electrons, and chlorine can accept one electron in its outer shell.. The number of electrons that an atom of an element has in its outermost shell determines the chemical formula of the compounds formed by atoms such elements. ...
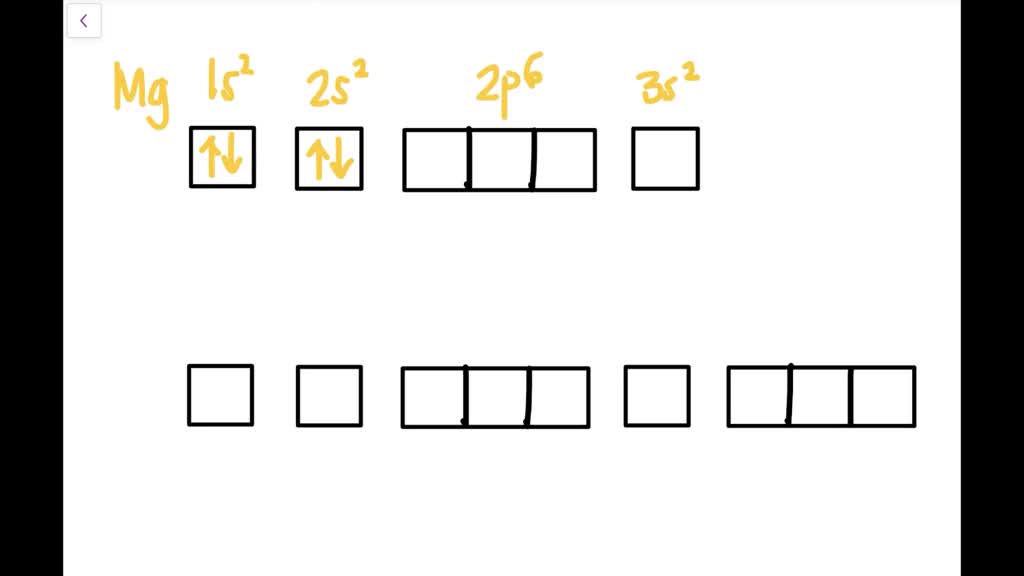
a) The atomic number of magnesium is 12, and its electronic configuration is as follows, 1s 2 2s 2 2p 6 3s 2.Therefore, the orbital diagram for magnesium can be drawn as,
A) Core electrons are the easiest of all electrons to remove. B) All of the above are true. C) Core electrons effectively shield outer electrons from nuclear charge. D) Outer electrons efficiently shield one another from nuclear charge. E) Valence electrons are most difficult of all electrons to remove.
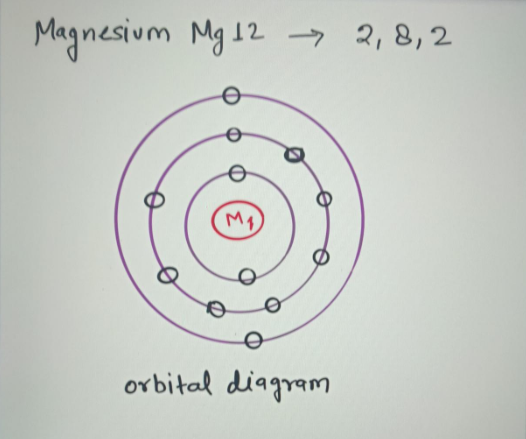
41 orbital diagram of magnesium. The Bohr Model of Magnesium (Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
Oxygen electron configuration is 1s2 2s2 2p4. The eighth element in the periodic table is oxygen. The period of oxygen is 2 and, it is a p-block element.
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
Give orbital diagram of the following: magnesium chloride, Medium. Open in App.
Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
Orbital diagram. Electron arrangement that uses arrows. Magnesium. What atom matches this electron configuration?1s2 2s2 2p6 3s2. 1s2 2s2 2p4. Which is the electron configuration for an oxygen atom? 2. What is the maximum number of electrons that an orbital can have? 5. How many d orbitals are there in a given sublevel? 3.
The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
In the case of magnesium oxide (MgO) both the participating atoms are in the form of ions that are not directional, so they cannot have molecular geometry, hybridization, and molecular orbital diagram. The non-compliance to covalent properties and ionic bonding can further be explained with the help of the electronegativity concept.
This is a diagram of the Periodic Table. As we can see, Mg belongs to group 2 and has an atomic number of 12 whereas Cl belongs to group 17 and has an atomic number of 17. Mg has 2 valence electrons whereas Cl has 7 valence electrons. The total number of valence electrons in a molecule of magnesium chloride = 2*1 + 7*2 = 16.
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
Write the electron configurations for mathrmmg and mathrmar using both spdf notation and orbital b 2
Still magnesium In chemistry, excited state means that an electron from an orbital absorbs incoming radiation or a photon that corresponds to its energy level and hence is elevated to a higher energy level. Hence, when an element is excited, only electrons are concerned, thus not affecting the identity of the element. In order for an element to transmute or decay into another element, such as ...
Draw the abbreviated orbital diagram for the iron ion in [Fe ... A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively. A graph of the successive ionization energies of magnesium is shown below.
1.11.2021 · Note 1: If you want the valence electrons of all the 118 elements, then visit this article: Valence electrons chart for ALL ELEMENTS (Where I have shown the valence electrons using images). Note 2: If you want a periodic table with valence electrons labeled on it, then visit this article: Periodic table with Valence electrons (labeled on it) (From this article, you can also …
Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital. Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape. Mg2+ has an electronic configuration ...
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
The orbital diagram uses up or down arrows to symbolize the electron. Answer and Explanation: 1 Magnesium is a metal that possesses an atomic number of 12. An atomic number of 12 indicates that...
The orbital notation of magnesium is 1S2 2S2 2P6 3S2. orbital is indicated by a line and can contain two electrons that are drawn as up and down arrows. What is an orbital diagram? An orbital...
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2.
Magnesium atoms form ionic compounds with hydrogen. ... This article gives an idea about the electron configuration of hydrogen and orbital diagram, period and group, valency and valence electrons, bond formation, hydrogen compound formation, application of …
In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We'll also look at why Magnesium forms a 2+ ion and how the electron con...
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 …
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
Orbital Diagram For Magnesium - Evaluation E Coli Inhibition By Plain And Polymer Coated Silver half life half life symbol t 1⁄2 is the time required for a quantity to reduce to half its initial value the term is monly used in nuclear physics to




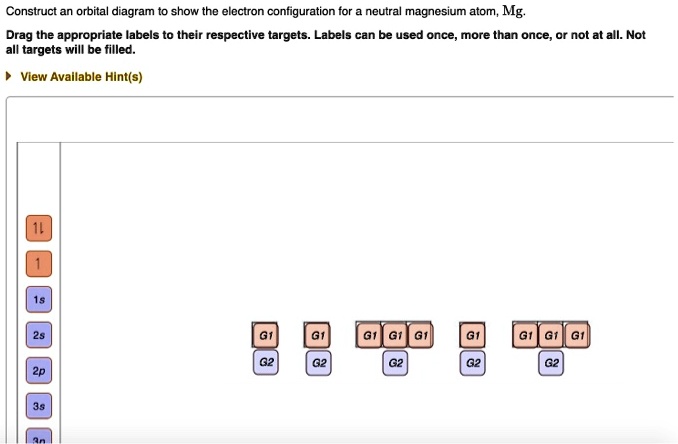




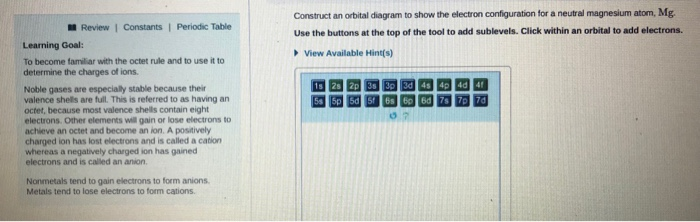






![6] (a) Write an orbital diagram for the ground state of Magnesium ...](https://img.homeworklib.com/images/b7f46805-3b3a-4cf6-8603-c907004a6c53.png?x-oss-process=image/resize,w_560)


0 Response to "36 orbital diagram for magnesium"
Post a Comment