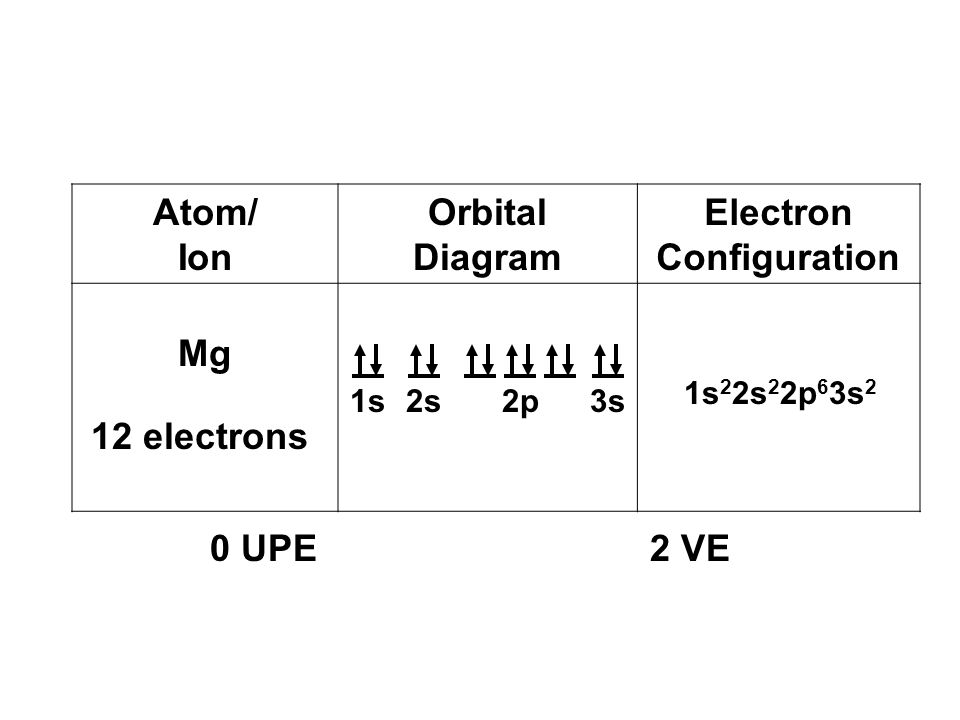
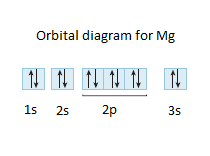
40 orbital diagram for mg
E. A 1s orbital can be represented as a two-dimensional circle centered around the nucleus of an atom. 3. When completing the orbital diagram for the element silicon, which of the following statements is correct? A. There are no electrons in the 3d sublevel. B. There are no electrons in the 3s sublevel. C. The 2p sublevel is not full. D.
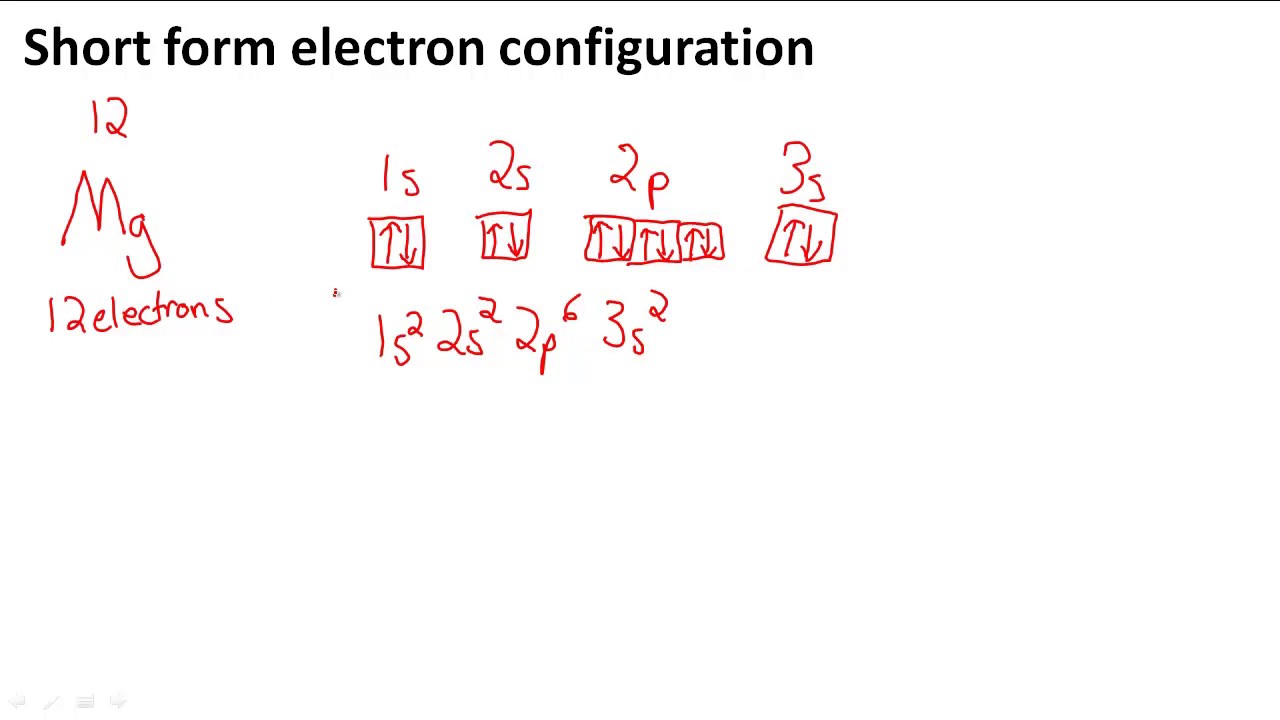
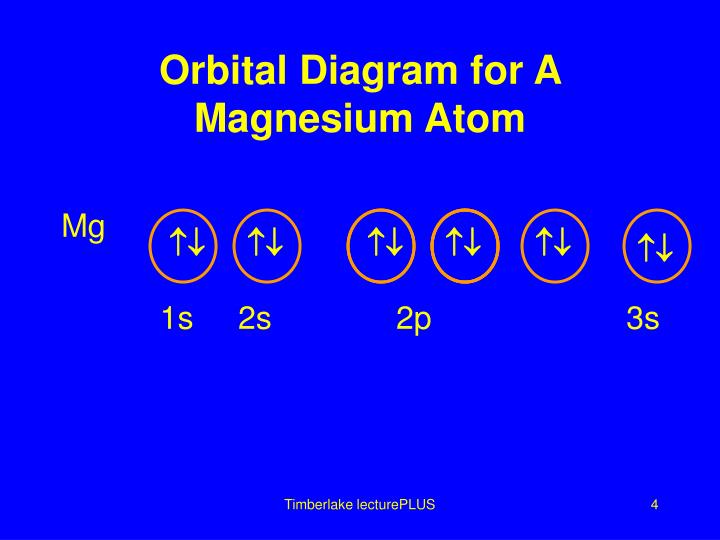
To write the orbital diagram for the Magnesium atom (Mg) first we need to write the electron configuration for just Mg. To do that we need to find the number...
PART B - ORBITAL NOTATION Use the patterns within the periodic table to write orbital notations (use arrows) for the following atoms. Symbol # e- Orbital Notation 7. Mg 8. P 9. V 10. Ge 11. Kr 12. N
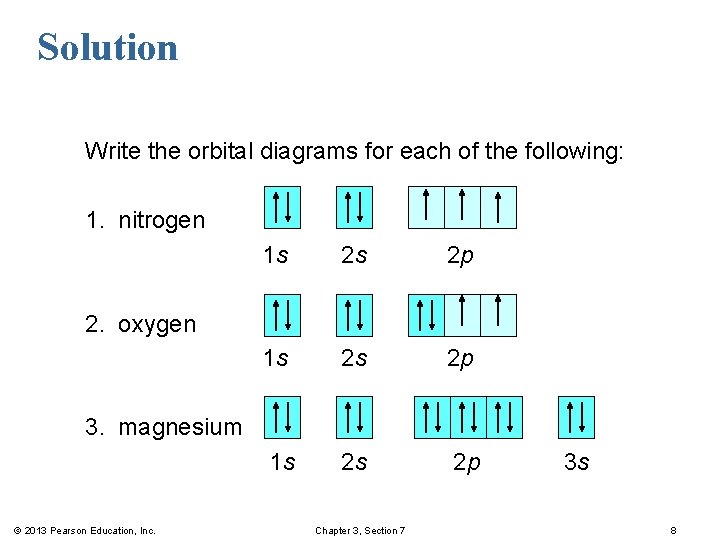
Orbital diagram for mg
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
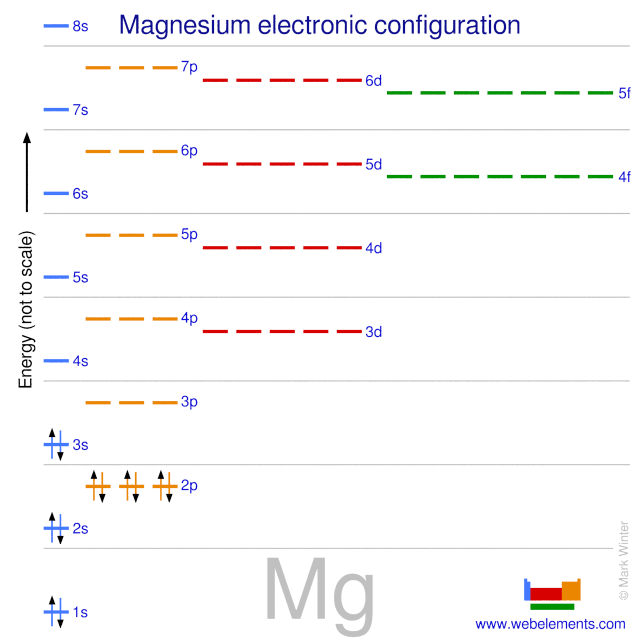
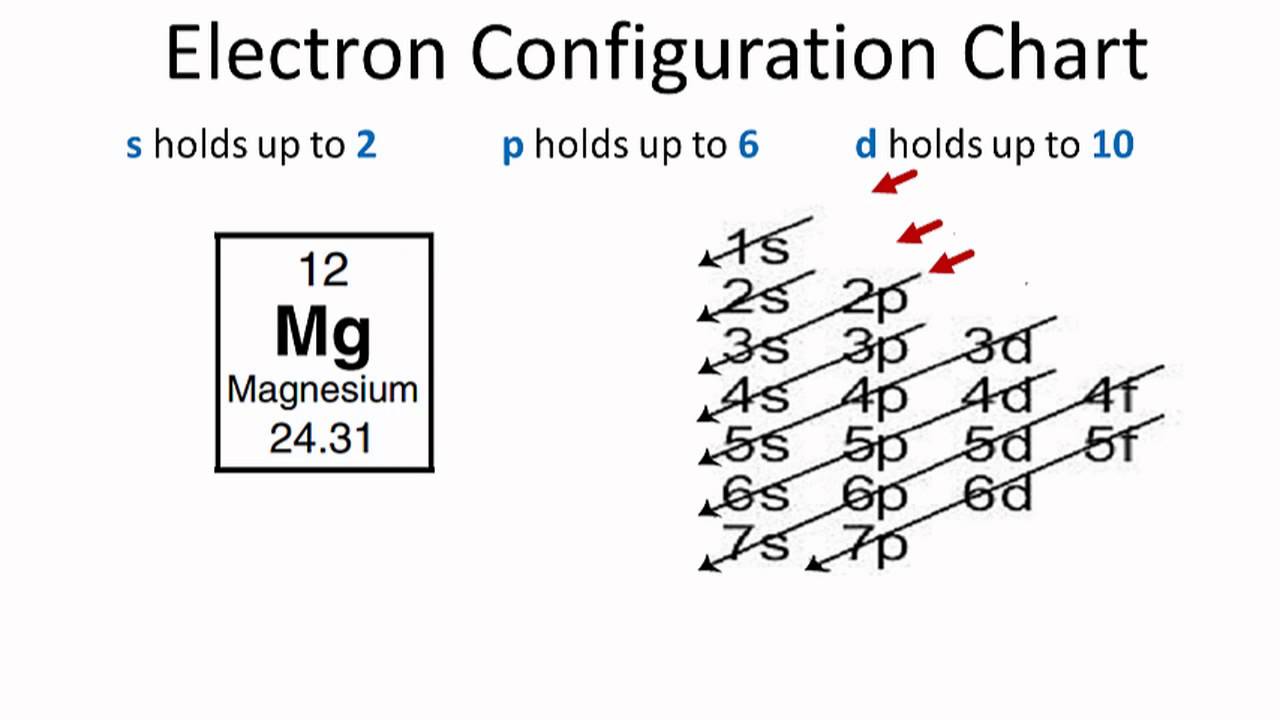
We examine electron configuration with following examples. Example: Helium 2. 1s 2. Where; 1 is the principal quantum number or energy level (shell) s is the sub-level or sub shell (Capacity of s sub shell is 2 electron) 2 shows the number of electrons in the s sub shell. Example: Chlorine 17.
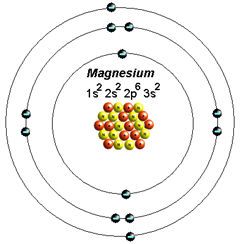
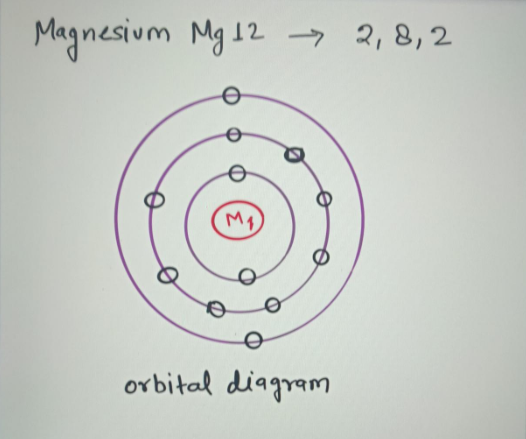
The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
Orbital diagram for mg.
Mg—> Mg²+ + 2e-Magnesium Cation Mg2+ Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital. Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape.
Determine the electron geometry (eg) and molecular geometry (mg) of CO3 2 A) eg=trigonal planar, mg=bent B) eg=tetrahedral, mg=trigonal pyramidal C) eg=tetrahedral, mg=tetrahedral D) eg=tetrahedral, mg=trigonal planar ... Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ ...
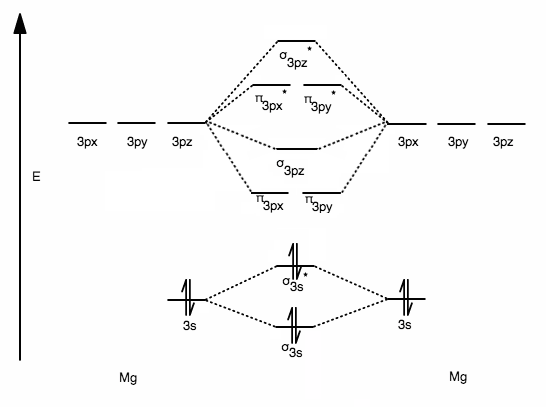
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
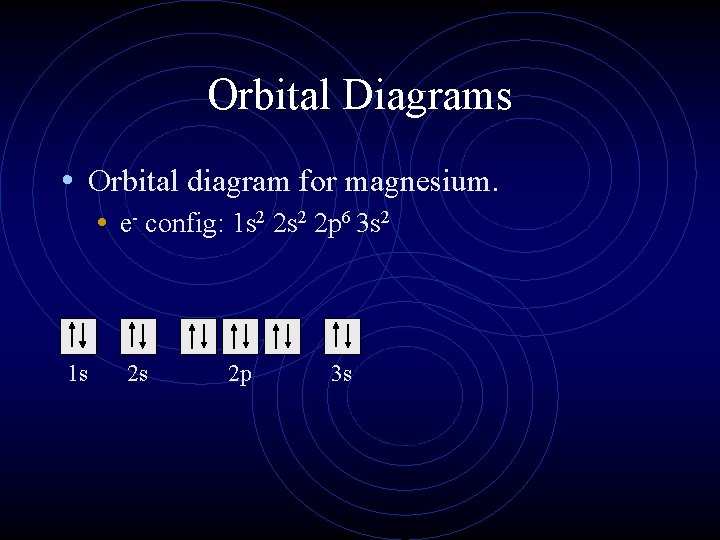
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s.
diagrams. For C the gap between the 2s and 2p orbitals is small, this is why C forms sp hybrids. However for all the other elements to the right of C such as N, O, F the sp gap is larger and gets increases long the periodic table, thus for F the gap is very large. Put the 2s and 2p AOs on the diagram taking note of the
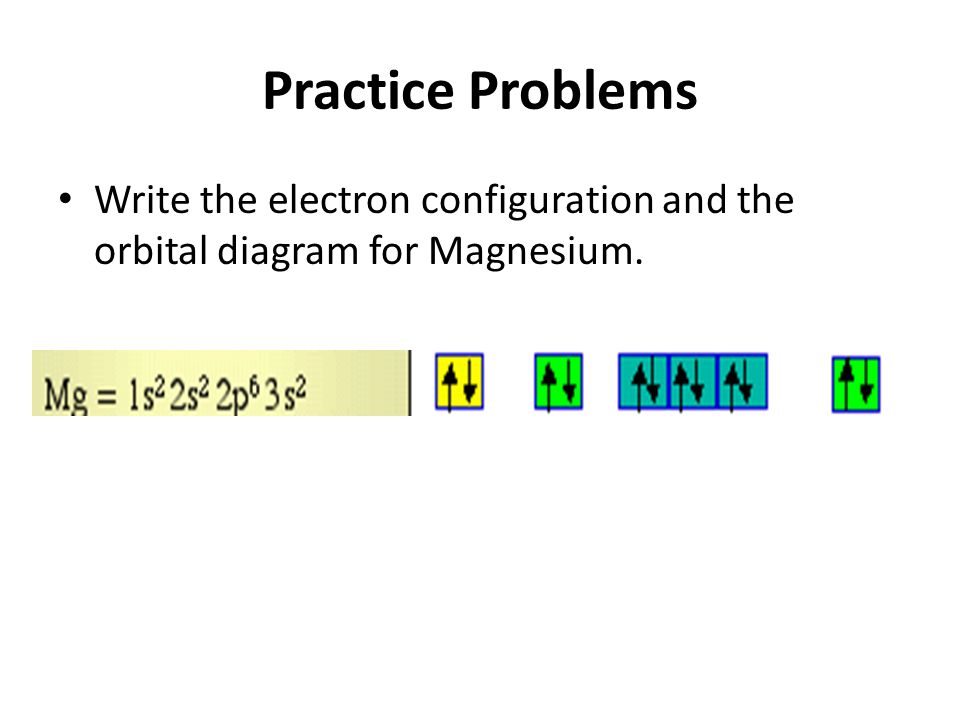
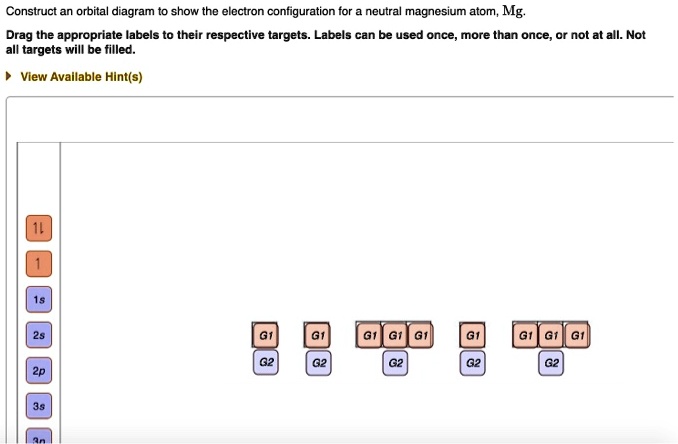
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, MgMg.Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled.
41 orbital diagram of magnesium. The Bohr Model of Magnesium (Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
Step by Step: Electron Configurations and Electron Orbital Diagrams Electron Configurations Ex. 1) Mg: 1s 2 2s2 2p6 3s2 ↑↑↑ 1 = 1. st. layer (row #), s = orbital type , power of 2 = the 2 electrons in the 1s orbital **Move the Helium box next to Hydrogen (above Beryllium.) See the periodic table below.
The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Likewise, what does mg2+ represent? Mg2+ is a Mg atom that now has the same number of electrons as a noble gas. It achiveved this by giving up ...
Part A - Orbital Diagrams . Use the patterns within the periodic table to draw orbital diagrams. Symbol Orbital Diagram (Don't do Nobel Gas Configuration) Mg P Ge Li Reminder of electron configuration rules: Aufbau Principle: Electrons occupy lowest energy levels first. Pauli Exculsion principle: Each orbital contains up to 2 electrons.
Orbital Diagram For Magnesium - Molecules Free Full how do u know the orbital diagram for mg best answer mg has 12 electrons we will fill them from lower orbitals to higher one orbital needs 2 electrons to fill in 1s one orbital 12 Orbital Diagram For Magnesium - Condensed Matter Free Full
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
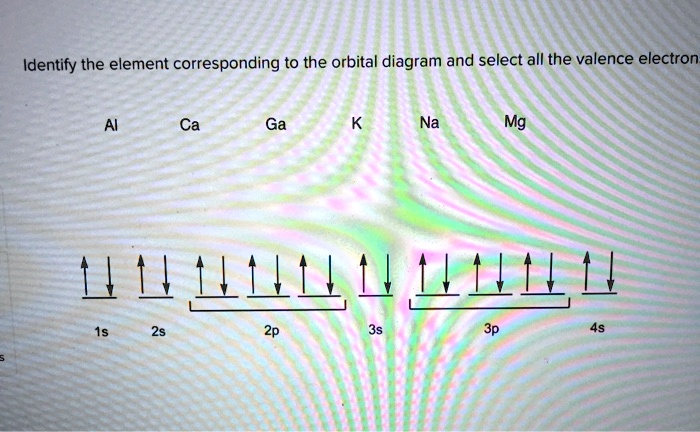
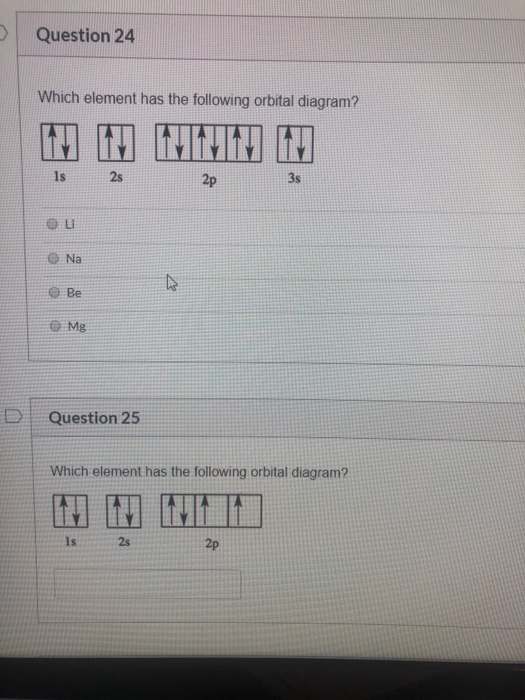
What element does the following orbital diagram represent? Mg (magnesium). What element does the the following orbital diagram represent? V (vanadium). Solutions for Chapter 8 Problem 4SAQ. Problem 4SAQ: Choose the correct orbital diagram for vanadium. step-by-step solutions; Solved by professors &. Oxidation States, +5,2,3,4.
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
a. (a) H - Mg - H is linear in its electron domain and molecular geometry. (b) The Mg atom has 2 electrons in its 2s orbital; these electrons are paired. Without promotion to an sp excited state, Mg has no unpaired electrons available to form bonds with hydrogen' (c) s-p (d) - )p hyrd "n HJ d K/il H /'7 5.
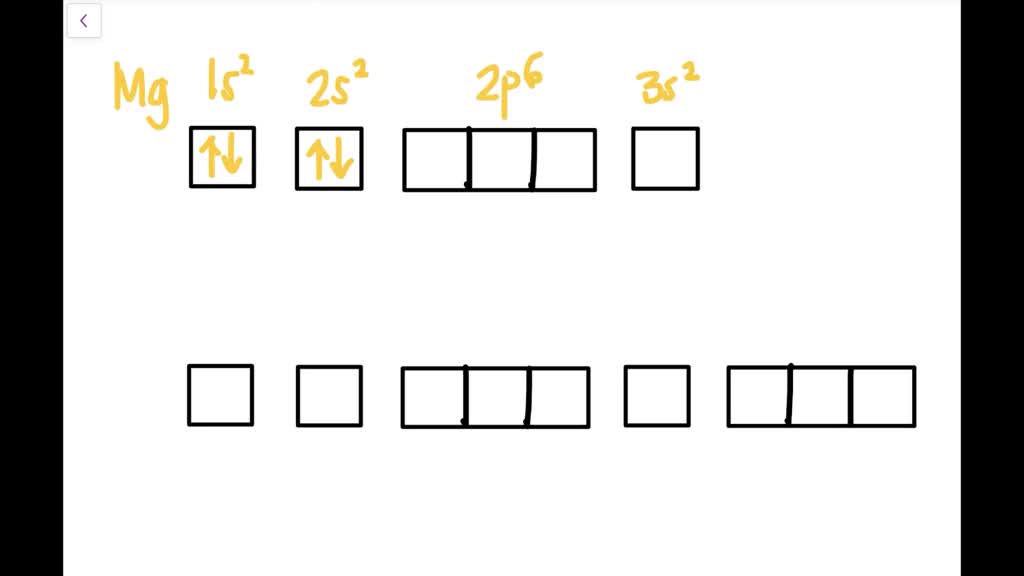
a) The atomic number of magnesium is 12, and its electronic configuration is as follows, 1s 2 2s 2 2p 6 3s 2.Therefore, the orbital diagram for magnesium can be drawn as,
The orbital notation for magnesium is: Magnesium is in the third row and second column of the Periodic Table. what is the orbital notation of magnesium and silicon? Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion (Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2.
What is the orbital diagram and electron configuration of magnesium? We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around ...
Start studying Honors Chemistry Unit 4 Test. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s^2 2s^2 2p^1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). Write the condensed electron configurations for the Mg atom.
















![6] (a) Write an orbital diagram for the ground state of ...](https://img.homeworklib.com/images/b7f46805-3b3a-4cf6-8603-c907004a6c53.png?x-oss-process=image/resize,w_560)


0 Response to "40 orbital diagram for mg"
Post a Comment