38 lewis dot diagram for iron
Lewis Structures for Polyatomic Ions. When writing dot structures for polyatomic ions, you must remember to add or subtract the amount of electrons represented by the charge. When writing polyatomic ions, you must include the structure inside brackets, [ ], with the charge outside the bracket
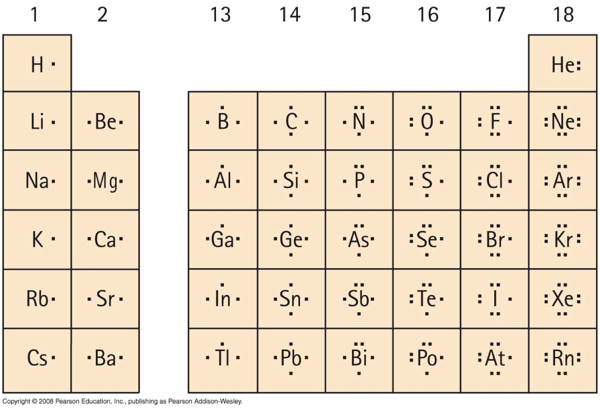
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
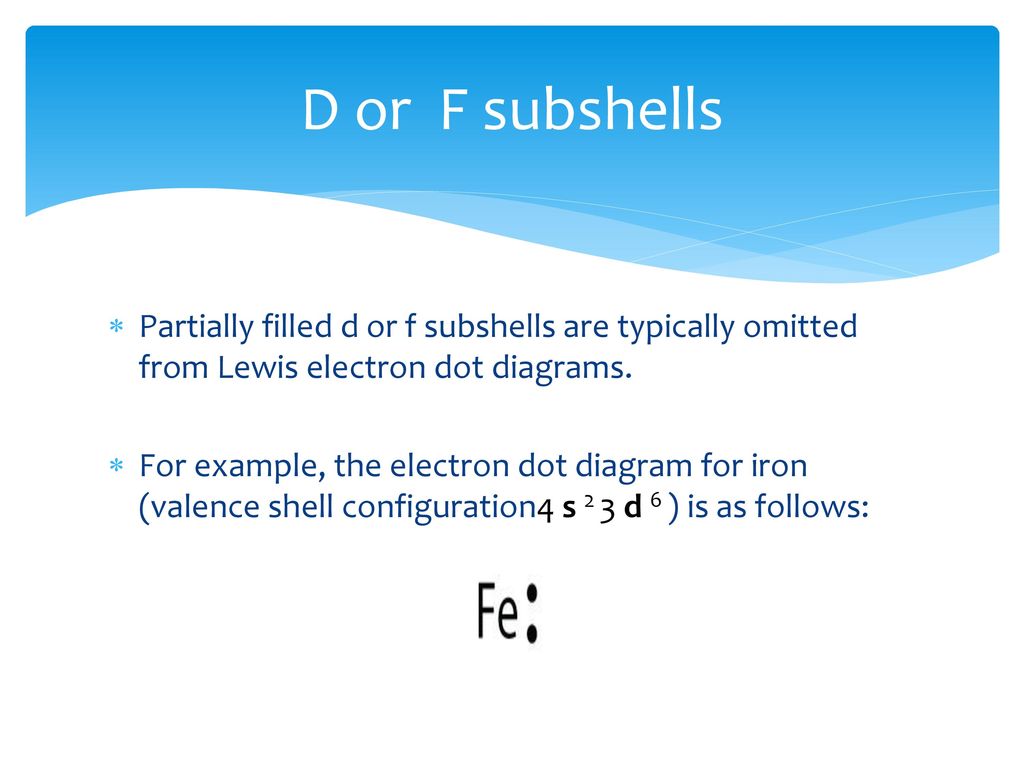
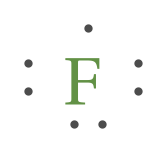
The Lewis dot diagram for Iron is the letters FE with seven dots around it, with no more than 2 dots on each side. Iron is not a part of the halogen family. Good try! However, the electron...
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
Lewis dot diagram for iron
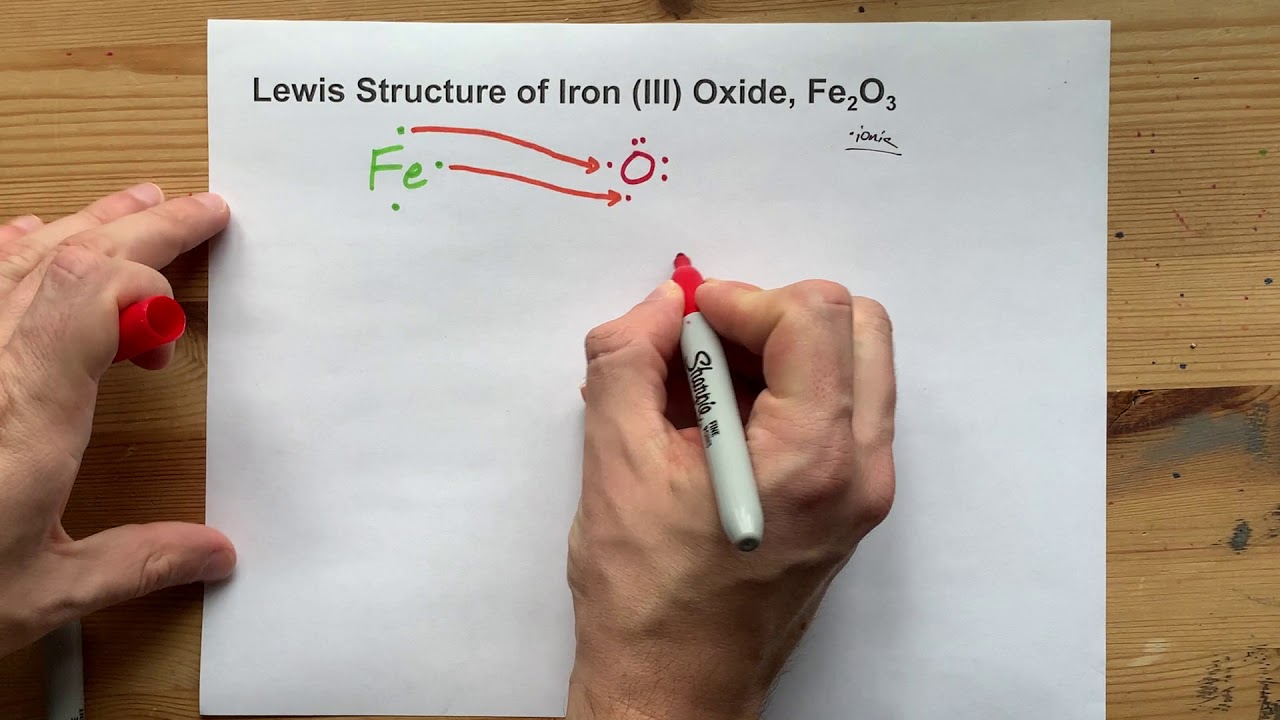
Draw two Lewis (electron dot) structures for BrO3- [1] Structure II —> does not follow octet rule ... Lewis structure with formal charges closet to 0 provides the greatest stability for the structure. Structure II has FC of 0 and structure I has FC of 2+ ... Draw the abbreviated orbital diagram for an iron atom using the arrow-in-box notation ...
Lewis Structure of Iron (II) Oxide, FeO. Iron (II) oxide’s Lewis Structure is among one of the easiest to draw. The iron atom, because it has a +2 charge in this compound, is drawn with two valence electrons – and since it is a metal, it wants to give them away (“lose them”). Oxygen, by contrast, is a non-metal with six valence ...
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
Lewis dot diagram for iron.
If you are asked to write the Lewis Structure of Iron (Fe) you'll first need to find the number of valance electrons for Iron. One of the challenges in writi...
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
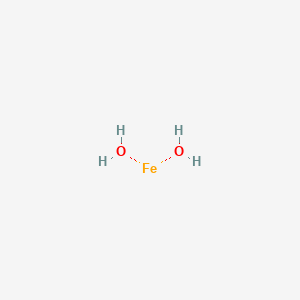
A step-by-step explanation of how to draw the Fe(OH)2 Lewis Dot Structure.For Fe(OH)2 we have an ionic compound and we need to take that into account when we...
To draw lewis dot structures, you first must know the number of valence electrons the atom has. By writing Fe in a noble gas configuration, you get [Ar] 3d6 4s2. By looking at this, since iron is a transition metal, you can see that iron technically has 8 valence electrons, but when bonding, it doesn’t always use all 8 valence electrons.





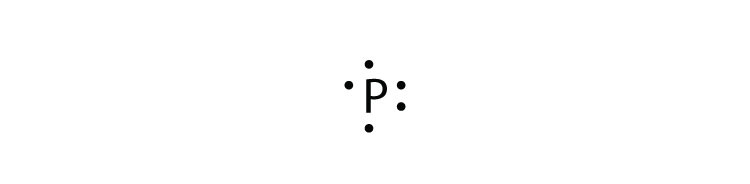
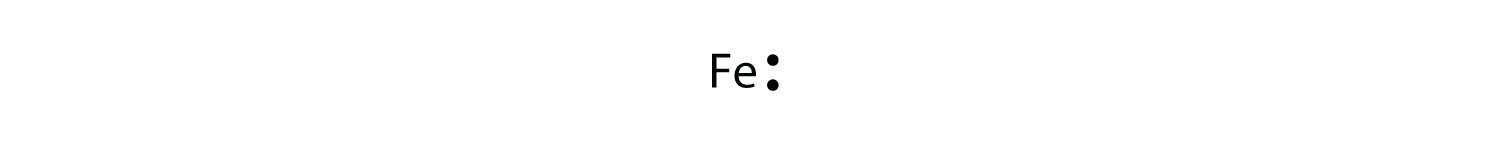














0 Response to "38 lewis dot diagram for iron"
Post a Comment