38 liquid vapor phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview. Common components of a phase diagram are lines of equilibrium or …
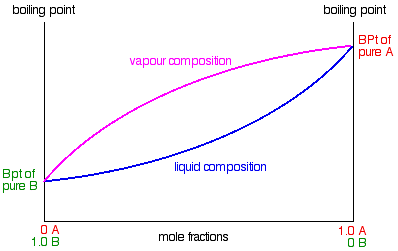
It explains how a phase diagram for such a mixture is built up and how to ... You get the total vapour pressure of the liquid mixture by adding these ...
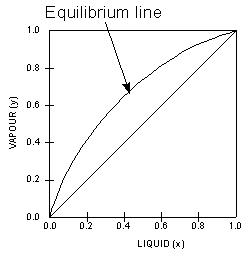
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if …
Liquid vapor phase diagram
3.3 Phase Diagram for Water Vapor: Clausius–Clapeyron Equation. The Clausius–Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius–Clapeyron Equation,
Phase Diagram for Water. Water is a unique substance in many ways. One of these special properties is the fact that solid water (ice) is less dense than liquid water just above the freezing point. The phase diagram for water is shown in the Figure below . Figure 13.26. Phase diagram for water. Notice one key difference between the general phase diagram and the phase …
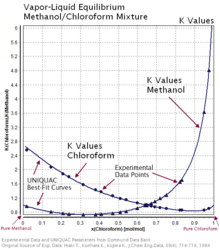
Consider a binary mixture of ethanol and water. Vapor-liquid equilibrium (VLE) data can be computed using the modified Raoult's law: , where is the vapor pressure, is the total pressure, and are the liquid and vapor phase mole fractions of the light component (i.e., ethanol) when , and finally, is the activity coefficient. You can vary the pressure to any value between and (i.e., …
Liquid vapor phase diagram.
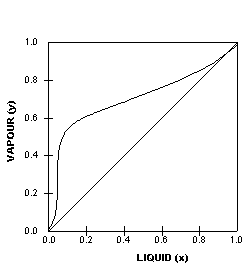
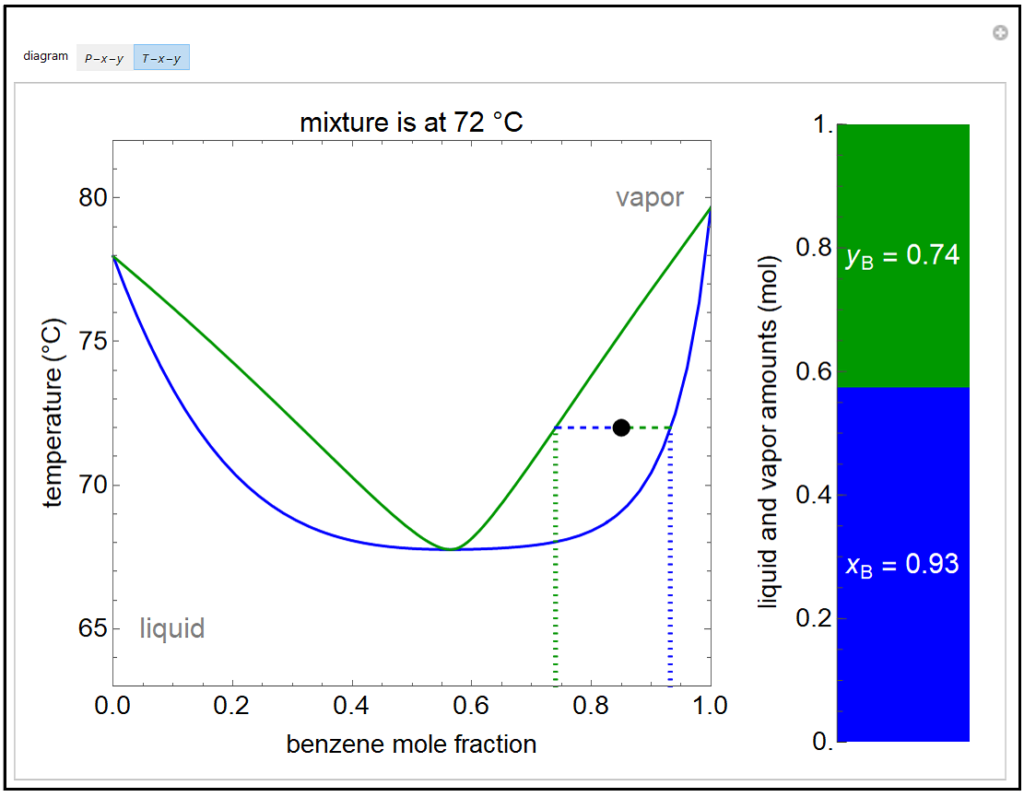
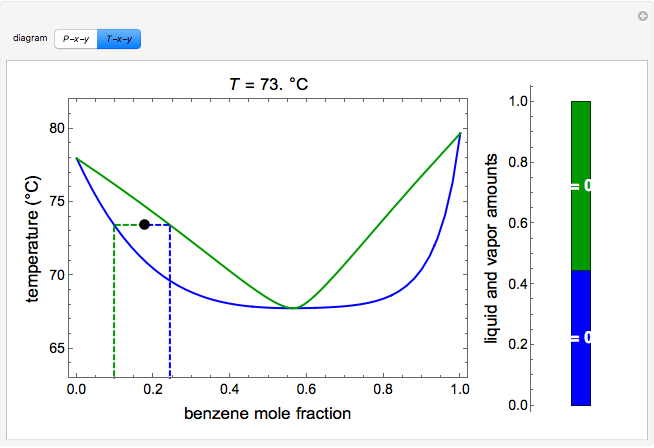
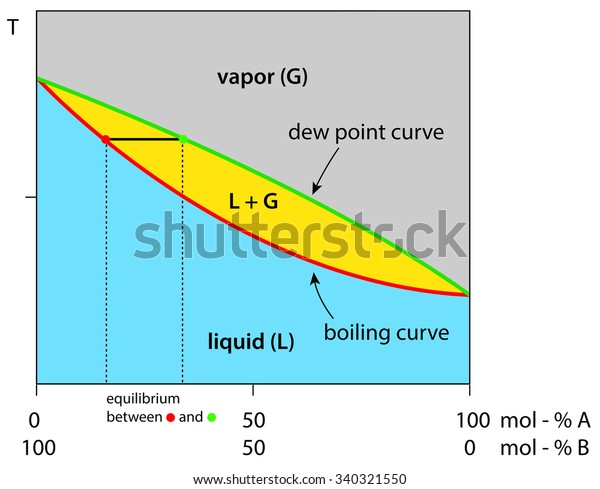
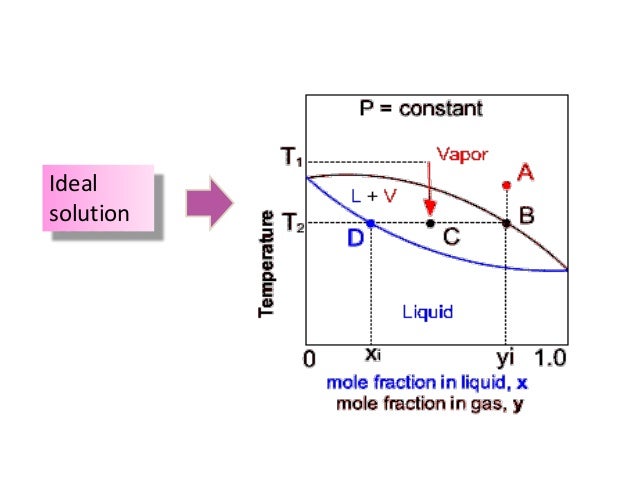
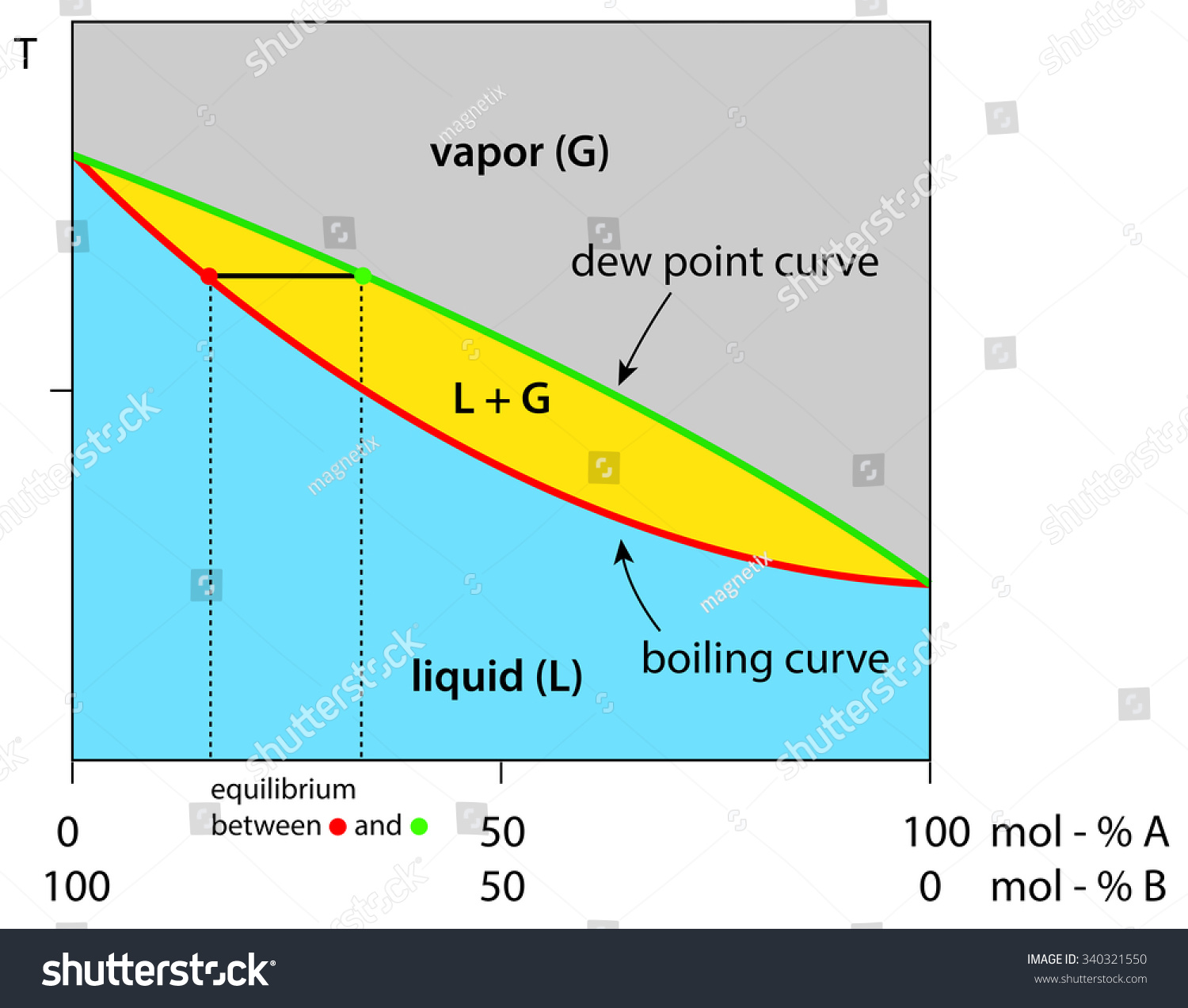
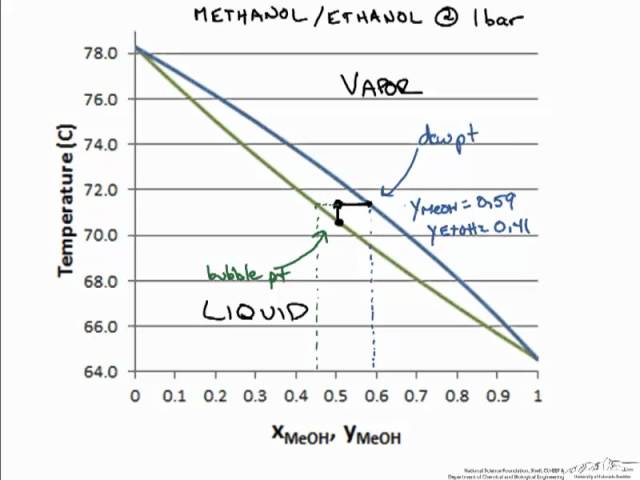
Application: Txy Diagram. Vapor/liquid equilibrium behavior of a binary mixture can be conveniently summarized in a Txy diagram. The Txy diagram depicts the solution to the equations . The second of these equations comes from setting the partial pressures of the indivdual components equal to the total pressure. Solving the first equation for then substituting …
Txy and Pxy diagrams — Multiple-Component Phase Equilibrium: Phase Diagrams ...
08/10/2020 · A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. The states of matter differ in the organization of particles and their energy. The main factors that cause phase changes are changes in temperature and pressure. At the phase transition, such as the boiling point between liquid and gas phases, …
Phase diagram of water Note: for H2O melting point decreases with increasing ... When molecules in the gas phase collide with the liquid surface, they loose energy and return to the liquid. At some point the rate of vaporization and the rate of condensation become equal and the system is at equilibrium. The partial pressure of the vapor above the liquid established at …
7 Nov 2017 — On the basis of ab initio Gibbs ensemble Monte Carlo simulations, we map the liquid–vapor phase diagram of water described by the RPBE ...SPECIAL ISSUE: This article is part of the Benj...
8 Mar 2021 — Oftentimes, it is desirable to depict the phase diagram at a single pressure so that temperature and composition are the variables included in ...
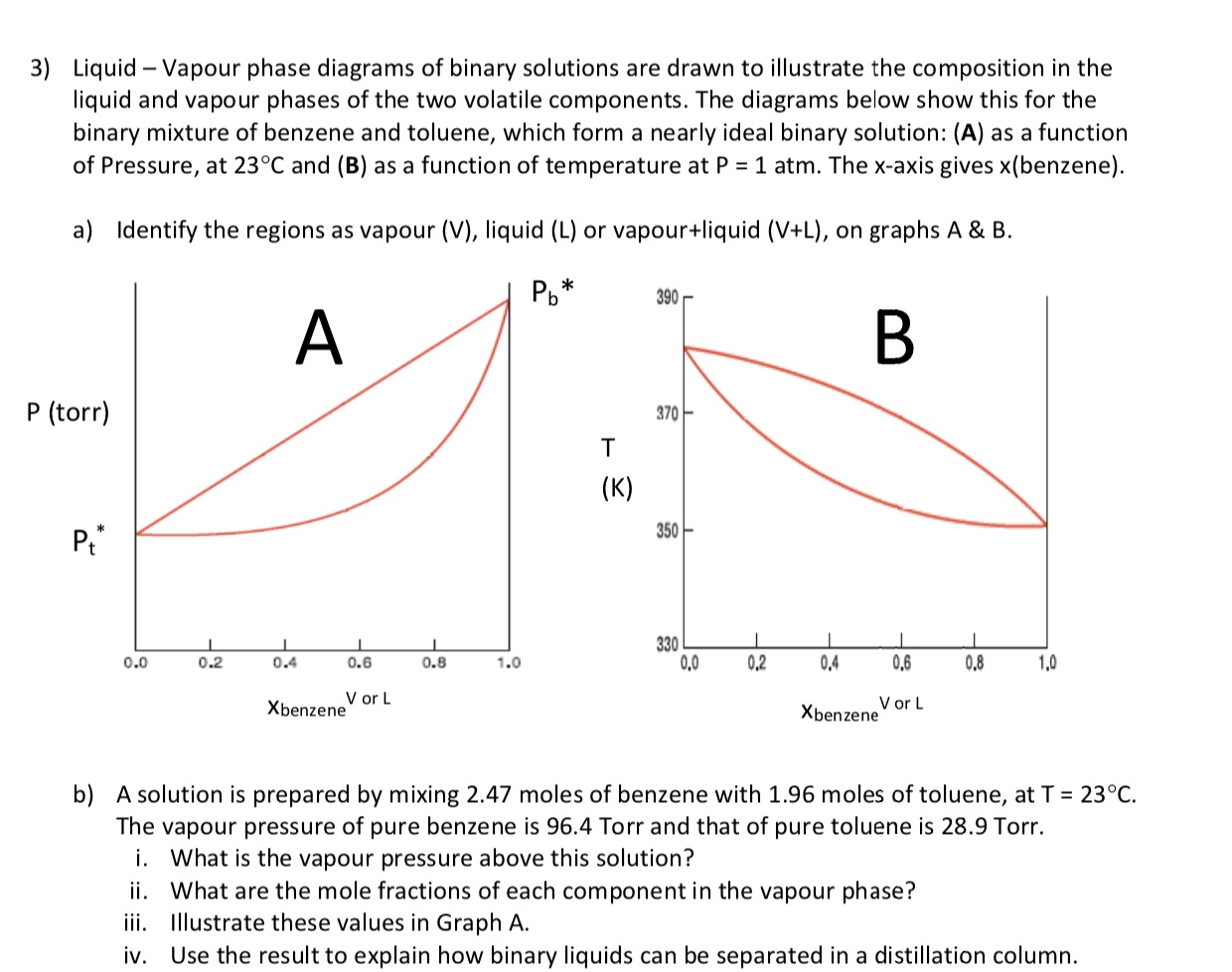
The vapor-liquid equilibrium (VLE) behavior of a benzene (C6H6)/toluene (C7H8) mixture is demonstrated in P-x-y and T-x-y diagrams. The blue line represents ...10 Sept 2016
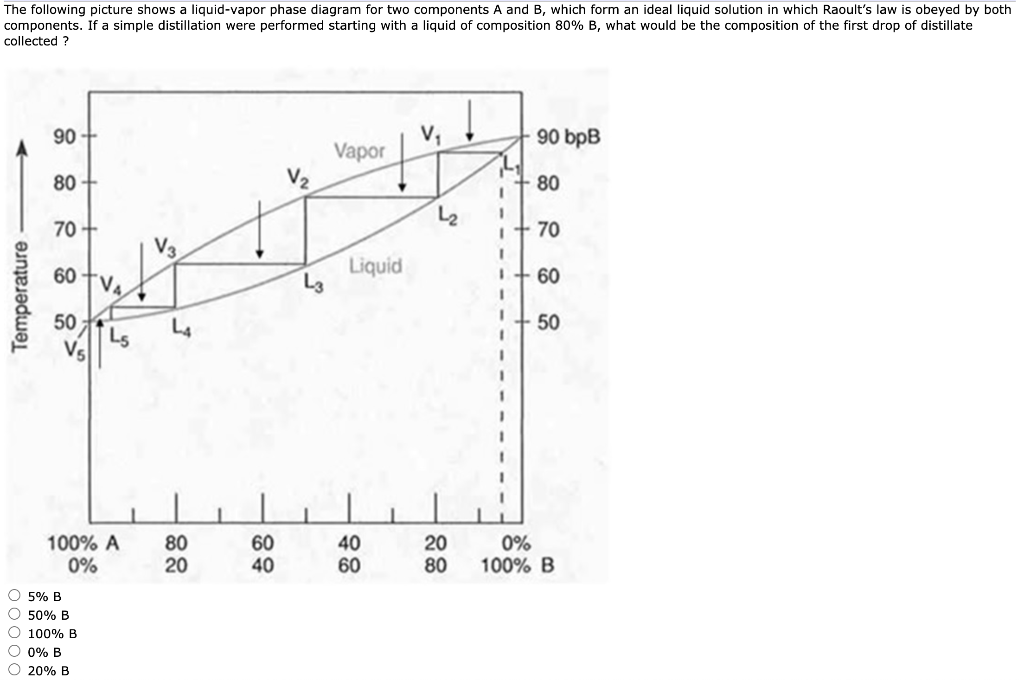
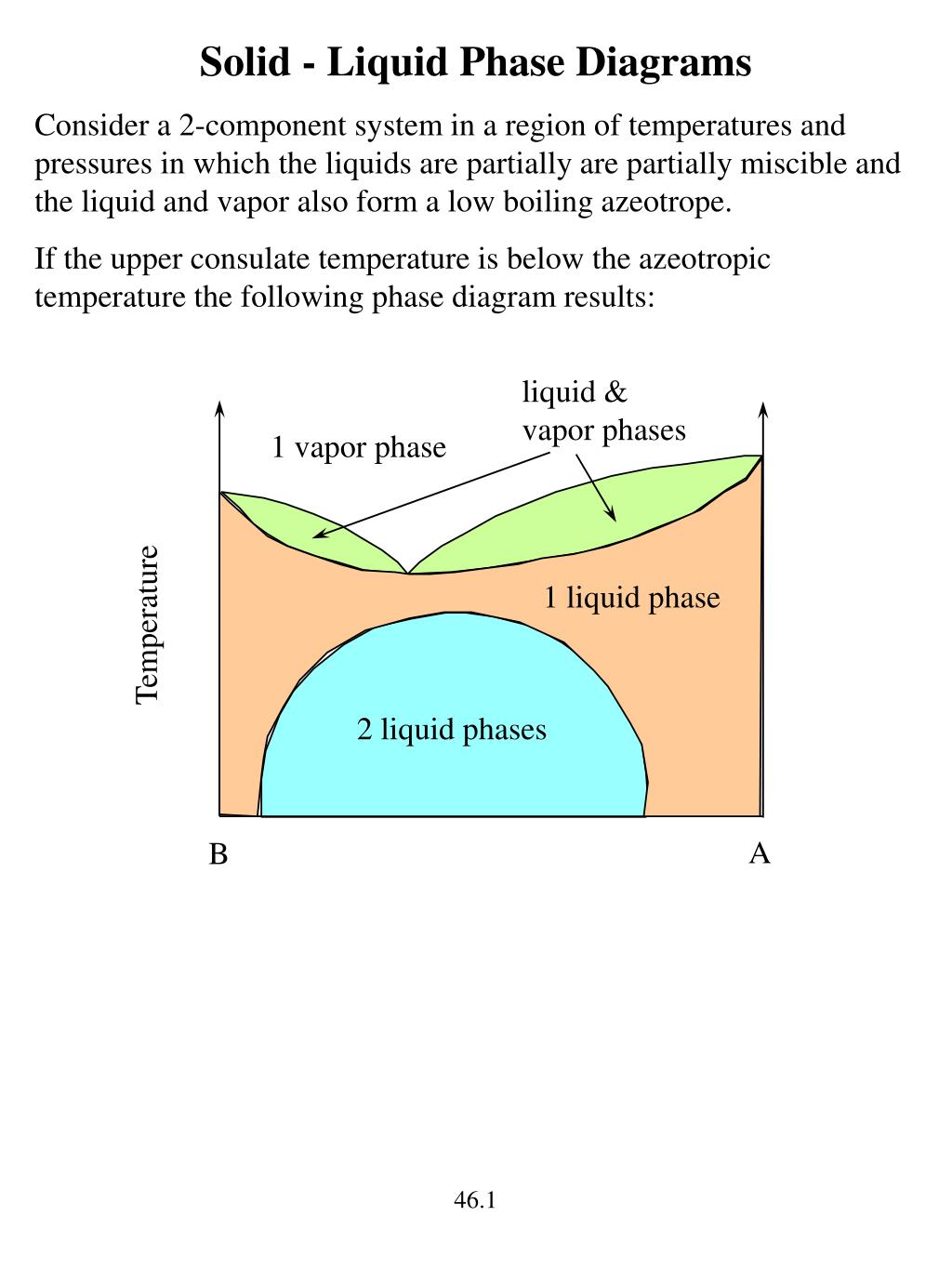
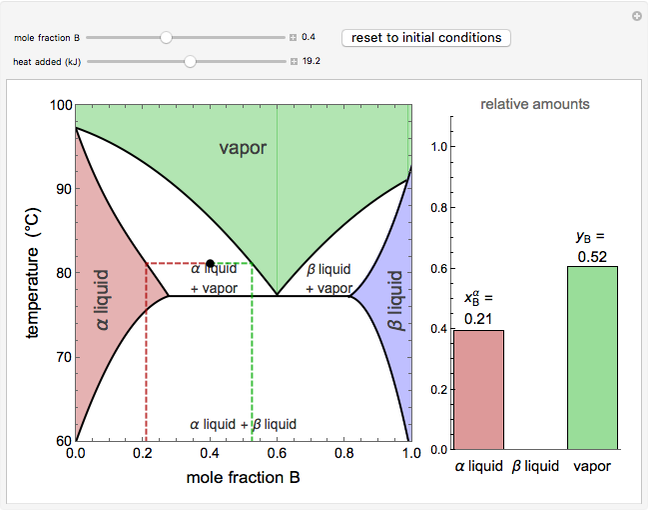
Construct a phase diagram using temperature versus mole percent composition. In the present case, there will be two compositions plotted at each equilibrium ...12 pages
The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid, and gaseous states of the …
5.3, and the common tangent construction then gives the equilibrium vapor and liquid compositions. The phase diagram depends upon the Gibbs energies of ...
13/08/2014 · Usually the solid phase is the densest. To be sure, look at the slope of the solid-liquid line. If it is decreasing, the liquid phase is denser, if it is increasing, the solid phase is denser. For example, the phase diagram of water has a negative solid-liquid line; the liquid phase of water is denser.












0 Response to "38 liquid vapor phase diagram"
Post a Comment