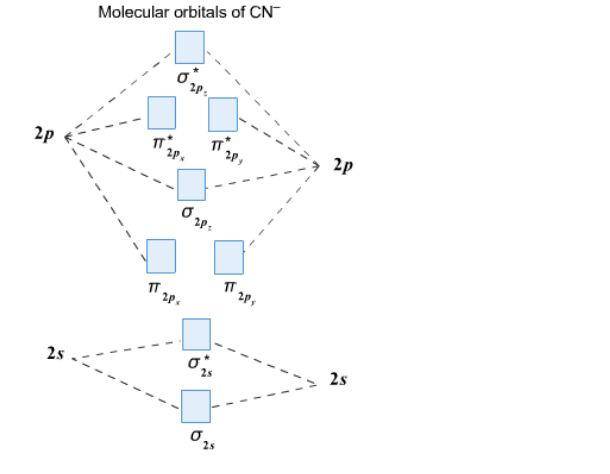
40 molecular orbital diagram practice
[Book 1 of The HEL Jumper](https://www.reddit.com/7oulr8) [Book 2 of The HEL Jumper](https://www.reddit.com/akws1r) \----- [Previous](https://redd.it/idztze) | [First](https://www.reddit.com/eo9svn) | [Next](https://redd.it/iqrcc1) | [Patreon](https://www.patreon.com/SabatonBabylon) Thanks to Big_Papa_Dakky, Darth_Android, bloblob, AMERICUH, The_Real_Jumper, Mr_Polygon, Krystalin, Damned_Thrice, Mamish, Vikairious, Sam_Berry, RedHawkdude, KillTech, LilLaussa, Daddy_Talon, Gruecifer, Gaelan_D... Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, ...9 pages
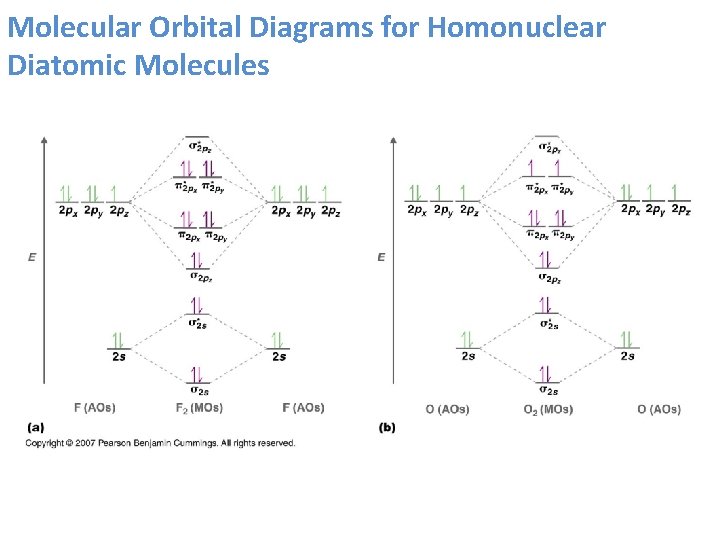
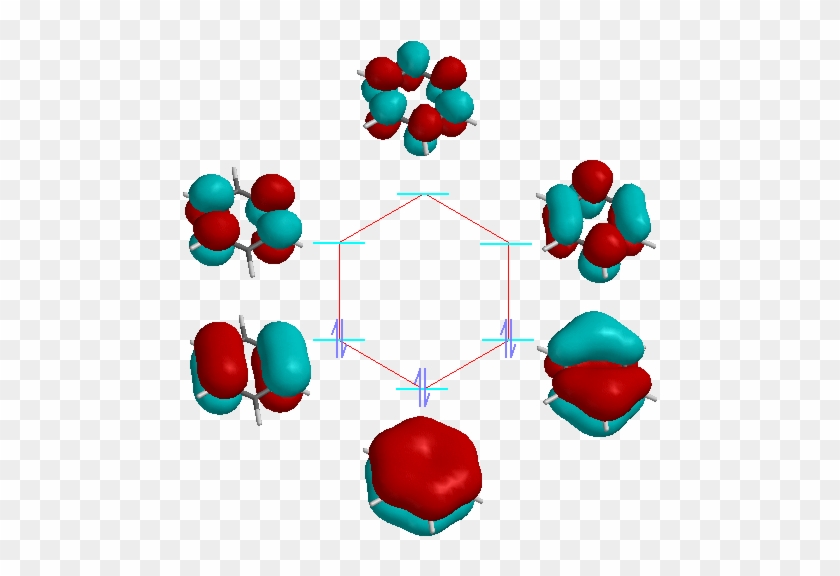
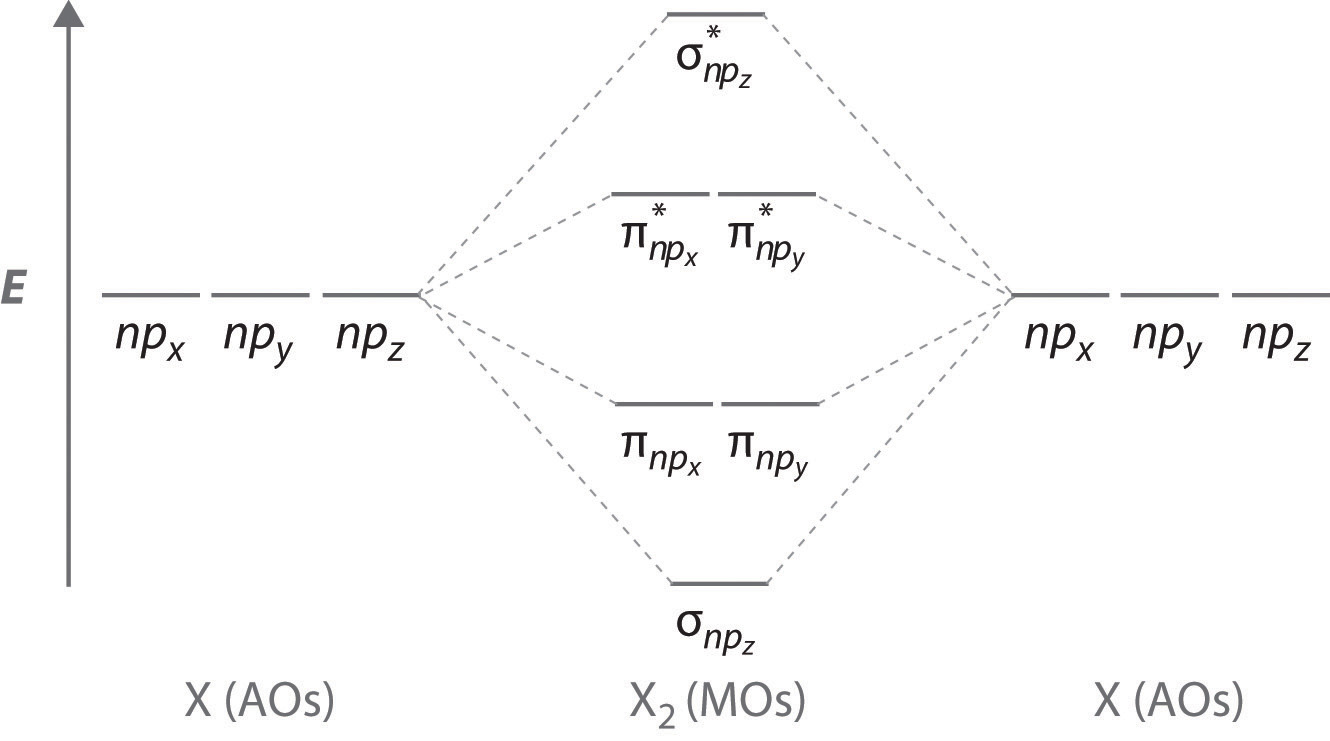
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z …

Molecular orbital diagram practice
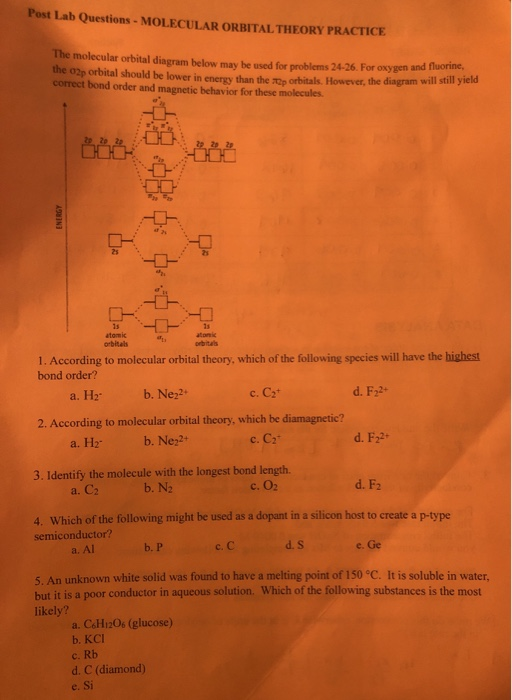
The electron configuration becomes 1σ22σ*21π4, the number of unpaired electrons is 0, and the bond order is 2. Other MO diagram constructions can give different ... However, the diagram will still yield the correct bond order and magnetic behavior for these molecules. 1. Refer to the Molecular Orbital diagram above.2 pages Molecular orbital diagram of two singlet excited states as well as the triplet ground state of molecular dioxygen. From left to right, the diagrams are for: 1 Δ g singlet oxygen (first excited state), 1 Σ + g singlet oxygen (second excited state), and 3 Σ − g triplet oxygen (ground state).
Molecular orbital diagram practice. Feb 15, 2015 — Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules. The site includes opportunities to practice ... CHEM 2000 Exercises and Practice Test Questions · 01 Reviewing Atomic Orbitals, Electron Configurations and Lewis Diagrams · 02 Molecular Orbitals of Homonuclear ... A little background: I am in my junior year of college working towards my BS in chemistry and hopefully moving on to grad school. I've taken two organic chemistry classes, one organic lab class (the higher lab class for chemistry majors vs the normal orgo lab class for non chemistry majors). I've taken one inorganic chemistry class and am currently in a class called advanced inorganic. I am also currently taking an inorganic lab class. I've also taken my general chemistry classes, analytical, an... www.calgarylearning.ca Hi, I am a recent PhD grad (Chemical Engineering. Over the past seven years I have served as a tutor, teaching assistant, lecturer, and lab instructor in a number of courses for high school and engineering students. My long term goals are towards Professorship and I am hence, offering tutoring to students for a number of University Mathematics, Physics Chemistry and Stats courses. These include courses as Kinematics, Work Energy Power, Rotation in Physics, Chemical Eq...
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron.The bond angles are cos −1 (− 1 ⁄ 3) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane (CH 4) as well as its heavier analogues.Methane and other perfectly symmetrical tetrahedral molecules belong to … ​ ## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance: This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as... HÜCKEL MOLECULAR ORBITAL THEORY ... Occupy the orbitals according to a stick diagram. At this stage, we note that from our N pz orbitals we will obtain N π orbitals. Further, ... To illustrate how we apply Hückel in practice, let’s work out the energy of The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital. He (Z = 2): 1s 2. The third electron goes into the next orbital in the energy diagram, the 2s orbital. Li (Z = 3): 1s 2 2s 1
\[Posted with moderator approval\] I'm creating a sort of sci-fi weird adventure radiodrama, told in the second person. Essentially a piece that starts as a meditation then goes off the rails and becomes a disorienting absurd trip. I have a "story-ified" version that will be turned into script format upon completion. Please let me know how I can improve it, I'm worried it's not engaging enough in its current state. Thanks! Reverse Meditation Episode 1: The Paramour Recorded from a space stati... Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals combine to form molecular orbitals. The total electrons associated with the molecules are filled in the MOT diagram. To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is: 03.03.2021 · Plus, get practice tests, quizzes, and personalized coaching to help you succeed. Try it now ... Molecular Orbital Theory: ... Phase Diagram of Water vs Other Substances: ... As a science communicator, I'm very interested in raising the science literacy of the general public. That's why I've created a series of general chemistry tutorials that cover the topics in a year of high school chemistry, but in as clear and concise a way as possible. There are quantitative practice problems for those who want to learn the math, but it's also for any person to simply watch in a relaxed way in order to absorb the content and expand their understanding. I genuinely believe it's ...
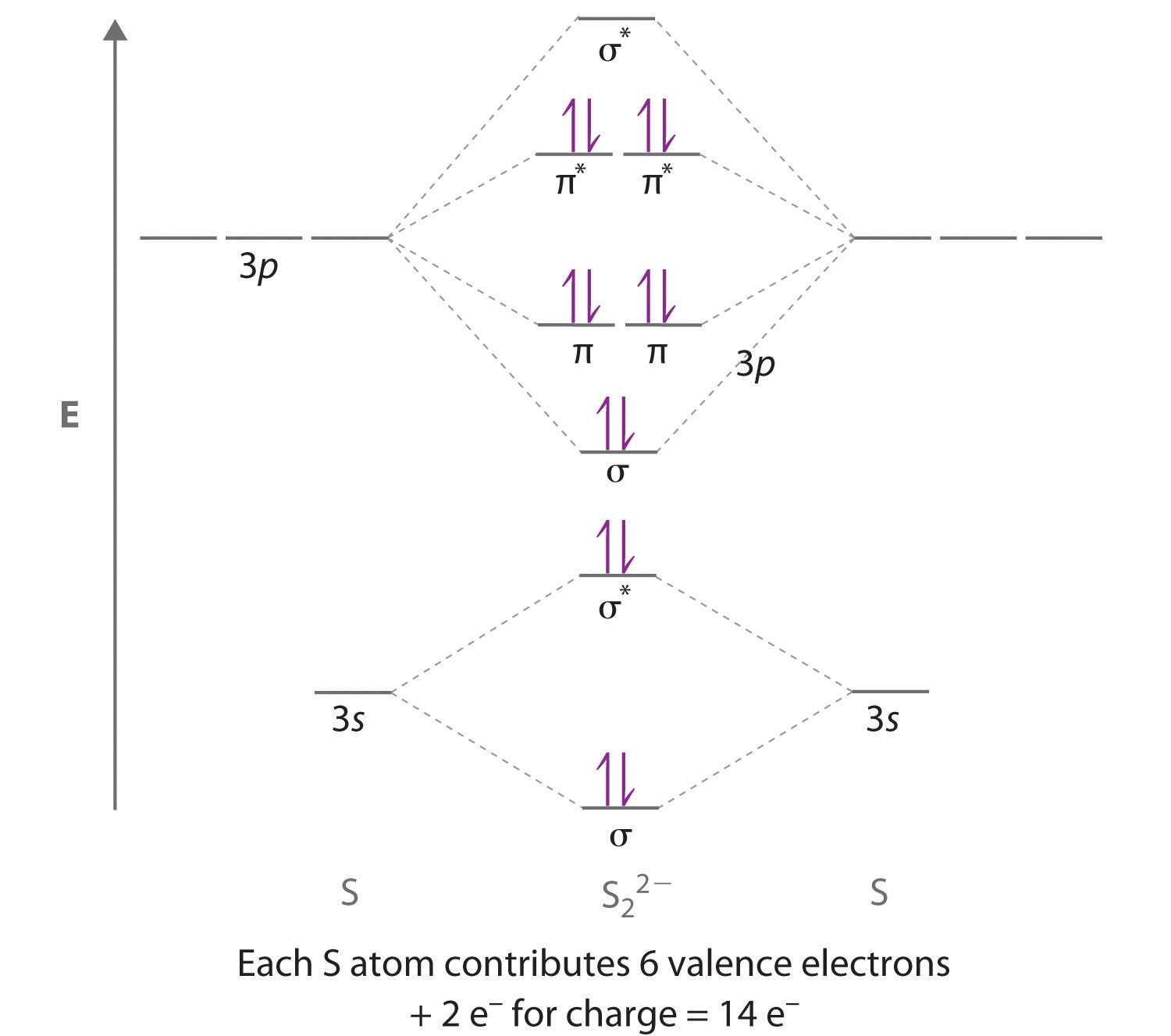
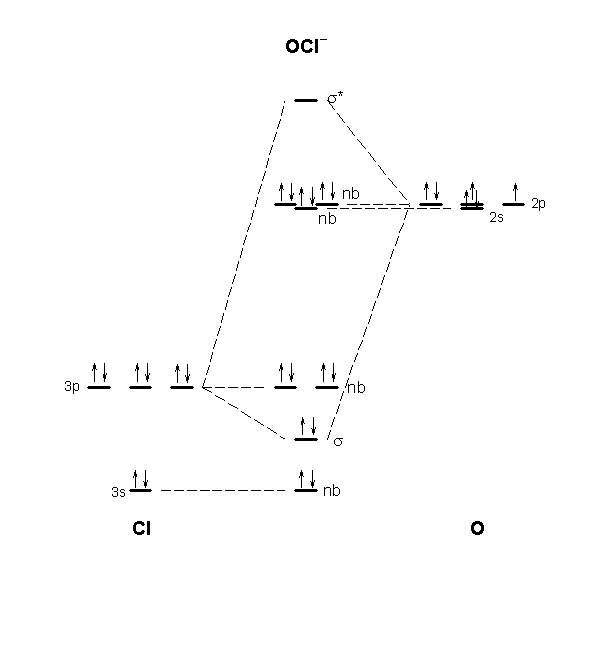
Practice Problems D: Orbitals & molecules ... Use a qualitative molecular orbital energy-level diagram to describe the bonding in S2. 2. − . What is the.4 pages
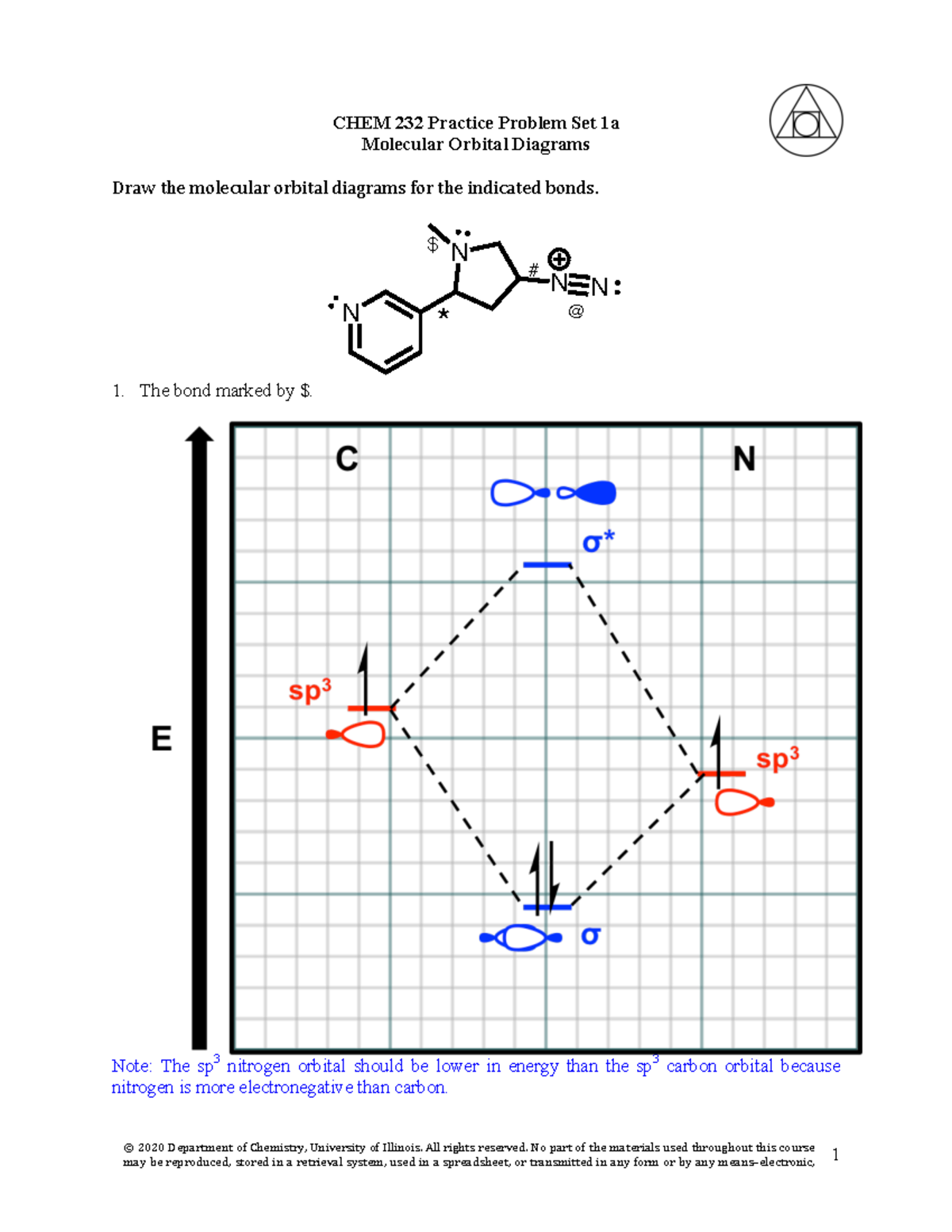
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
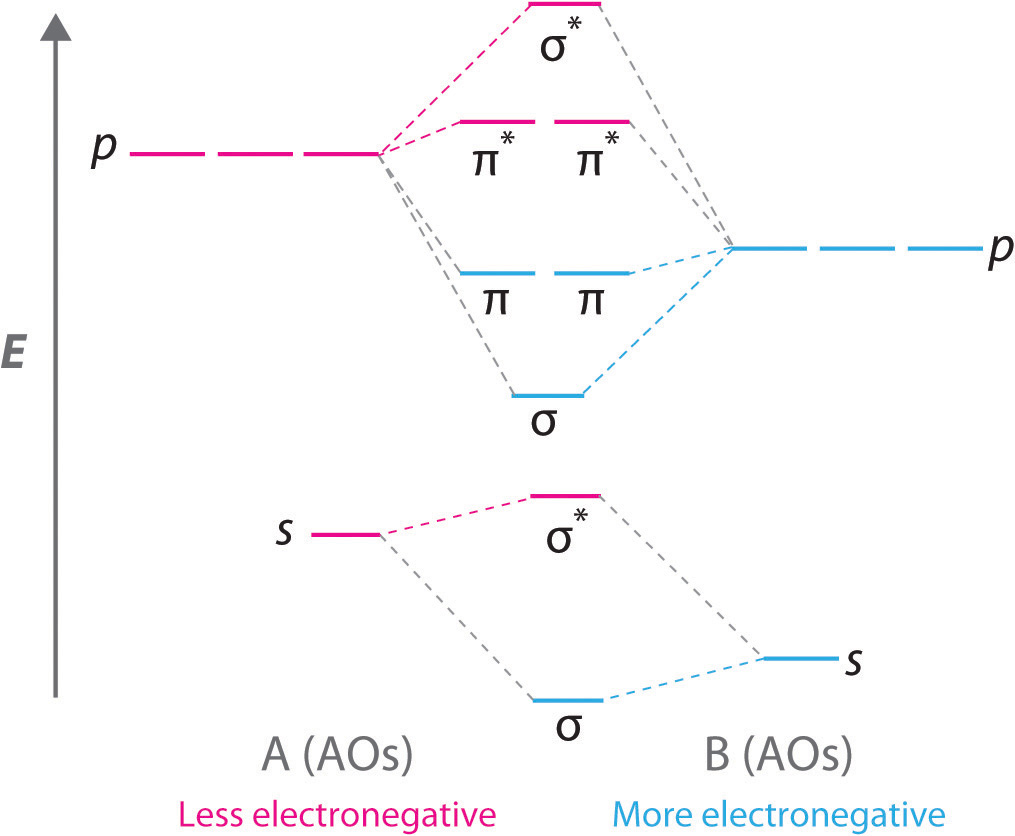
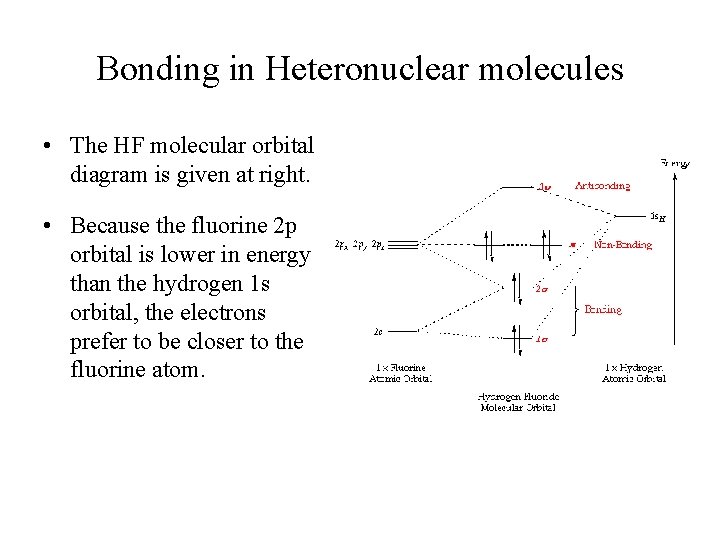
(c) Their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules. (d) The bonding molecular orbitals have more of the ...
Energy levels: Molecular Orbital Theory - revision 11m. Molecular Orbital diagram for CO 5m. UV/Visible spectroscopy 10m. Example of Beer-Lambert calculation 8m. Transitions relevant to UV/Vis Spectroscopy 15m. ... 2 practice exercises. Week 2 …
To answer such questions different theories and concepts have been put forward from time to time. In this fourth unit of class 11 chemistry, we can answer the above questions by learning Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory and Molecular Orbital (MO) Theory.
\[[https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic\_Table\_of\_Elements\_w\_Chemical\_Group\_Block\_PubChem.png](https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic_Table_of_Elements_w_Chemical_Group_Block_PubChem.png) \] or \[[https://ptable.com/#Properties](https://ptable.com/#Properties) \] In the last post, I mentioned the concept of activation barriers: reactions require an energy input to proceed from starting materials to a transition state even if there is a net re...
## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance:This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as protons and neut...
Mar 8, 2021 — Practice energy diagrams for molecular orbital theory. · Calculate the number of bonding and antibonding electrons in simple molecules.
As a science communicator, I'm very interested in raising the science literacy of the general public. That's why I've created a series of general chemistry tutorials that cover the topics in a year of high school chemistry, but in as clear and concise a way as possible. There are quantitative practice problems for those who want to learn the math, but it's also for any person to simply watch in a relaxed way in order to absorb the content and expand their understanding. I genuinely believe it's ...
Molecular orbital diagram of two singlet excited states as well as the triplet ground state of molecular dioxygen. From left to right, the diagrams are for: 1 Δ g singlet oxygen (first excited state), 1 Σ + g singlet oxygen (second excited state), and 3 Σ − g triplet oxygen (ground state).
However, the diagram will still yield the correct bond order and magnetic behavior for these molecules. 1. Refer to the Molecular Orbital diagram above.2 pages
The electron configuration becomes 1σ22σ*21π4, the number of unpaired electrons is 0, and the bond order is 2. Other MO diagram constructions can give different ...











0 Response to "40 molecular orbital diagram practice"
Post a Comment