37 lewis diagram for nh3
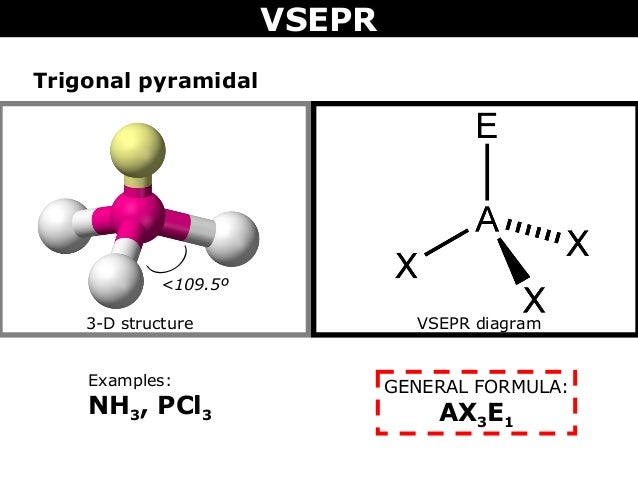
Nh3 Lewis Structure has 4 regions of electron thickness on all sides of the central nitrogen atom (3 bonds and one lone pair). These have a tetrahedral shape. These have a tetrahedral shape. The ensuing molecular shape or structure is like trigonal pyramidal with H-N-H angles of 106.7°. Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe ...
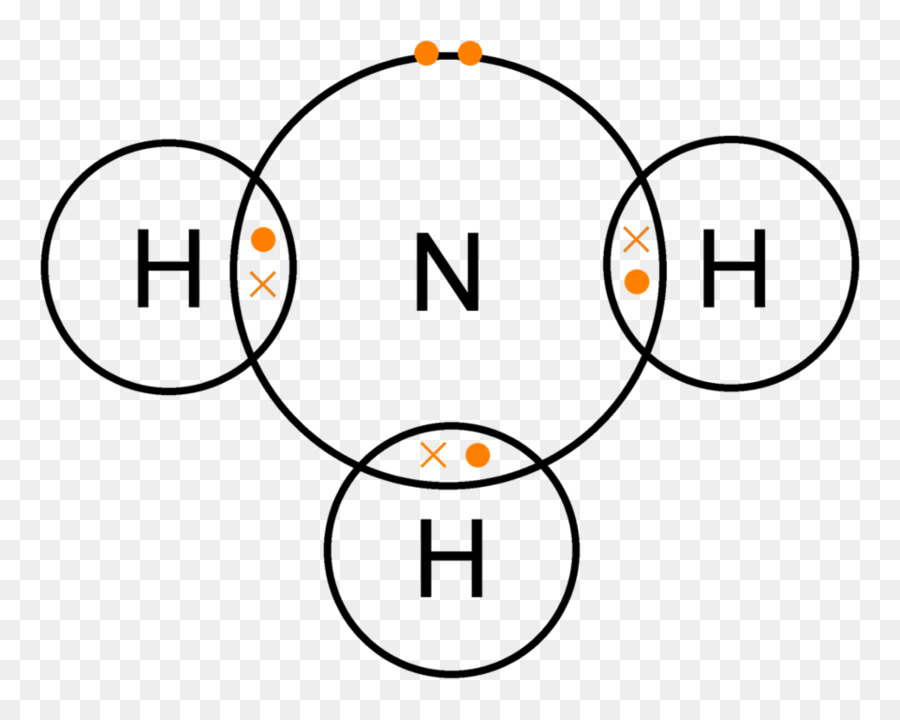
The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the molecules. Lewis Structure of NH3 The Lewis structure of nitrogen and hydrogen atom shows a total of eight valence electrons participating in a bond formation, to produce a single tetra-atomic NH3 molecule.
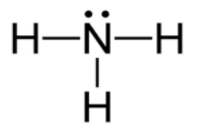
Lewis diagram for nh3
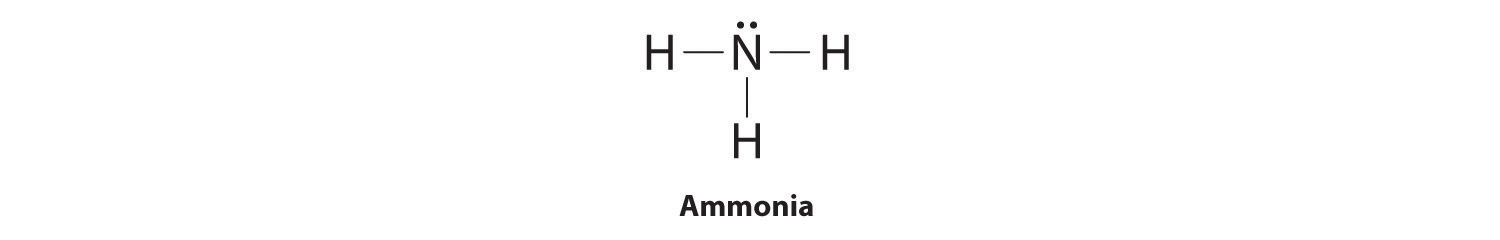
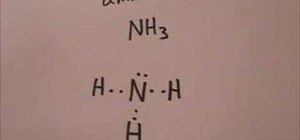
Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Lewis Dot of Ammonia · Back70 More Lewis Dot StructuresSince all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule Answer (1 of 3): One thing you know right off the bat is that nitrogen is in the middle. Hydrogen only has one valence electron, so it will just have the one bond. Draw N in the middle and draw the "H"s around it, one on each side and one either on the top or the bottom (it doesn't matter which)....
Lewis diagram for nh3. Solution for H-N-H A. The Lewis diagram for NH3 is: H. The electron-pair geometry around the N atom in NH3 is is There are lone pair(s) around the central atom,… January 4, 2021 - NH3 is the chemical formula of Ammonia. A positively charged polyatomic ion of Ammonium or NH4+ comes into existence when an Ammonia atom goes through the September 17, 2020 - The diagram of the NH3 Lewis structure is drawn using crosses and dots around the picture of an atom, by and large in pairs. Additionally, the lines show bond formation between the particles where it shows if a single, double bond, or triple bond has been formed. August 1, 2016 - Answer (1 of 3): Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to complete its octet.
By looking at the Lewis dot structure of ammonia NH3 molecule the shape of the ammonia molecule is described by which one of the following aTrigonal pyramidal bTshaped cSquare planar dTrigonal planar Draw the Lewis structure for NH3. Please include all nonbonding electrons. Question: Draw the Lewis structure for NH3. Please include all nonbonding electrons. This problem has been solved! See the answer See the answer See the answer done loading. Draw the Lewis structure for NH 3. Please include all nonbonding electrons. 5 May 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ... Answer for The Online Chemistry Classroom workbook - Drawing Lewis diagrams.
Mar 20, 2019 · Lewis Dot Diagram Of Nh3. Ammonia has the formula NH3. It's highly soluble in water because it's a polar substance. The Lewis structure for a molecule represents the total valence. The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Lewis Structures for NH3. In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Steps of drawing the lewis structure of NH3 is explained in detail in this tutorial. January 1, 2019 - Learn what the Lewis Dot Structure for NH3 is in this post by makethebrainhappy.
May 4, 2017 - Answer (1 of 17): NH3 (Ammonia) electron geometry is “Tetrahedral” but its molecular geometry is “Trigonal Pyramidal”. The best way to figure this out is to draw the Lewis structure. For the NH3 Lewis structure, calculate the total number of valence electrons for the NH3 molecule (NH3 ...
Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a ...25 Oct 2016 · Uploaded by Wayne Breslyn
January 10, 2019 - We show two ways to draw the NH3 Lewis structure, ammonia. We also have a handy video on the 5 things you need to know for general chemistry
The Lewis diagram for NHz is: 1 H The electron-pair geometry around the N atom in NHz is pyramidal There are 1 unshared pair (s) around the central atom, so the geometry of the NH3 molecule is trigonal pyramidal N B. The Lewis diagram for NOBr is: :0 Br: Recall that for predicting geometry, double and triple bonds count as only one electron ...
When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3's vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3).
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
HOME PAGE * KS3 SCIENCES * GCSE BIOLOGY CHEMISTRY PHYSICS * ADVANCED LEVEL CHEMISTRY · IGCSE AQA GCSE Chemistry A level Edexcel GCSE Chemistry A level OCR Gateway Science Chemistry A level OCR 21st Century Science Chemistry
Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of ammonia? Out of which 3 electrons of nitrogen form a covalent bond with hydrogen, with sharing of one electron of nitrogen and one electron of hydrogen. There are three such bonds.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
Apr 17, 2020 · Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side.
Craig Beals shows how to draw the Lewis Structure for Ammonia.This is a clip from the complete video: Covalent Bonding 2.1 - Drawing Lewis Structures
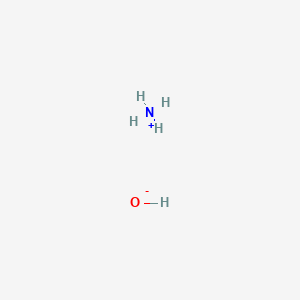
Apr 3, 2015 - A step-by-step explanation of how to write the Lewis Dot Structure for NH4+ (Ammonium Ion).For the NH4+ Lewis structure, calculate the total number of valenc...
I quickly take you through how to draw the Lewis Structure of Ammonia, NH3. I also go over hybridization and bond angle.
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.My Website: https://www.video-tutor.netPatreon: https:/...
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety. It also is a good example of a ...
A step-by-step explanation of how to write the Lewis Dot Structure for NH4+ (Ammonium Ion). For the NH4+ Lewis structure, calculate the total number of valen...
Transcript: OK, this is Dr. B. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let's mutiply ...
In NH4+ (ammonium ion) lewis structure, there are four sigma bonds around nitrogen atom. there is a +1 charge on nitrogen atom. Steps of drawing the lewis structure of NH4+ is explained in this tutorial.
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2.; 2; 6 years ago. most-viewed-thumbnail.
The Lewis structure of the tetra atomic ammonia (NH3) molecule has three single sigma bonds between the nitrogen and the hydrogen atoms. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the NH3 molecule.
Answer (1 of 3): One thing you know right off the bat is that nitrogen is in the middle. Hydrogen only has one valence electron, so it will just have the one bond. Draw N in the middle and draw the "H"s around it, one on each side and one either on the top or the bottom (it doesn't matter which)....
Lewis Dot of Ammonia · Back70 More Lewis Dot StructuresSince all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule
Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.

U.S. Representative John Lewis poses for a photo at Black Lives Matter plaza in Washington DC (IG: @clay.banks)

Caption reads, "[John Lewis speaking at a meeting of American Society of Newspaper Editors, Statler Hilton Hotel, Washington, D.C.] / [MST]." Original black and white negative by Marion S. Trikosko. Taken April 16th, 1964, Washington D.C, United States (@libraryofcongress). Colorized by Jordan J. Lloyd. Library of Congress Prints and Photographs Division Washington, D.C. 20540 https://www.loc.gov/item/2003688130/





















0 Response to "37 lewis diagram for nh3"
Post a Comment