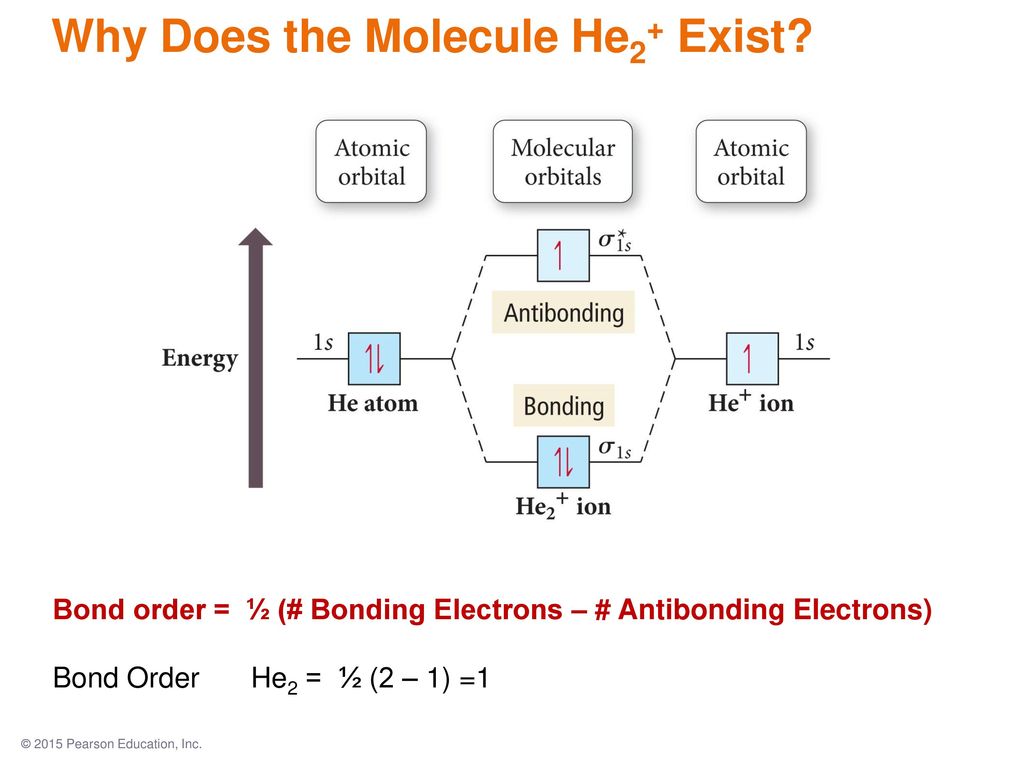
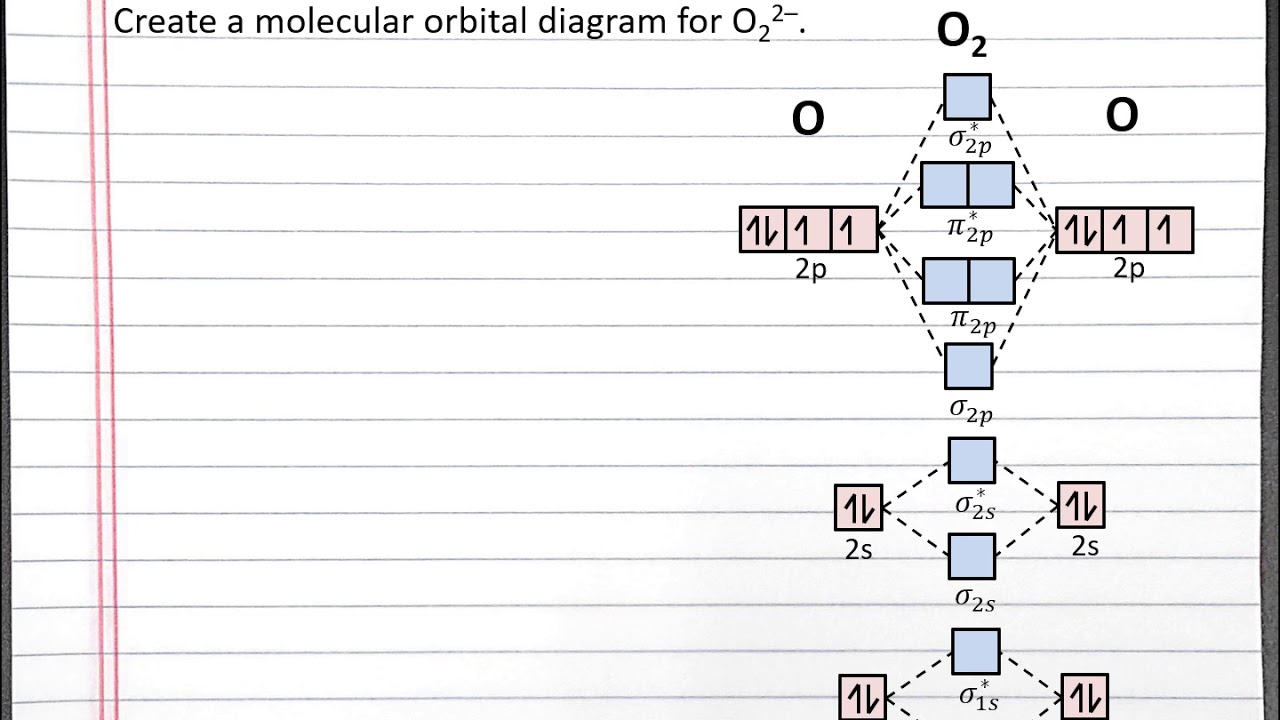
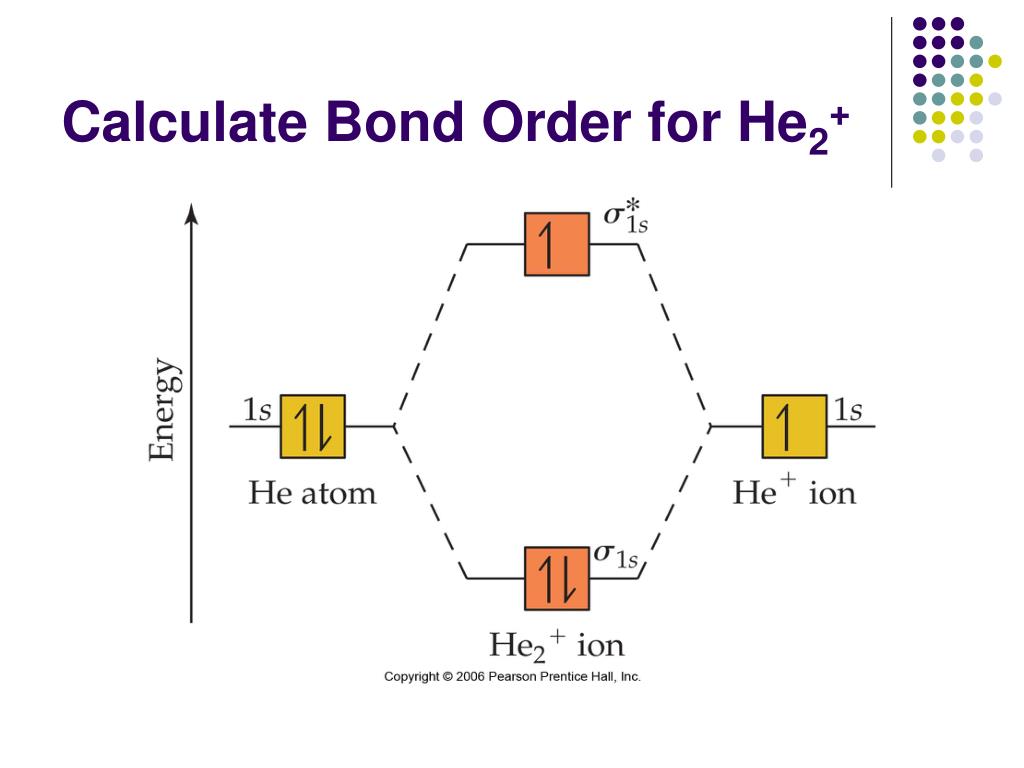
38 he2+ molecular orbital diagram
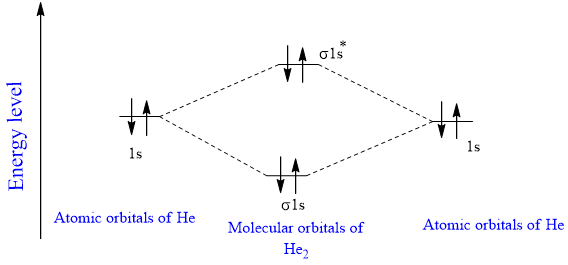
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h2 molecule is shown in figure a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear … In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo...

He2+ molecular orbital diagram
Molecular orbital theory of He2. confusingly showing for the energy diagram for He2 that the change in energy up to the anti-bonding orbital. He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above. Three electrons, two in sigma, one in sigma*. MO - Partial Overlap - Constructive or Destructive Hydrogen 1 and Hydrogen 2 combine to form a new molecular orbital. 결합성 분자오비탈 두 원자핵 사이에 전자가 발견된 확률이 높다. 쉽게 말해서... 한마디로 He2 분자는 존재한 수 없다는 이야기이다. Noble gas 가 왜 안정하고 , 반응하지 않는지 설명을 복잡하게도 햇다. 기본원칙 원자오비탈의 갯수 = 분자 오비탈의 개수... PEET 과수원 과학&수학... Notes Structure of nitrous oxide Keywords nitrous oxide Title Molecular shapes Caption Three-dimensional... Keywords VSEPR, molecular shapes Title Two Charge Clouds Caption Structures of carbon dioxide and hydrogen... Notes Although four charge clouds results in a tetrahedral electronic geometry, the molecular geometry is... orbital, molecular orbital, bonding, antibonding 07-16 Title MO Diagrams for H2 - ion and He2 molecule Caption...
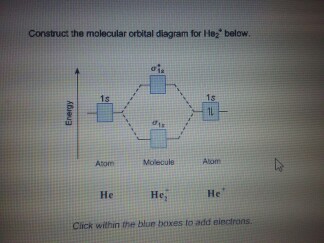
He2+ molecular orbital diagram. 약 20.9만 명의 구독자를 보유한 chemistNATE의 유튜브. 약 680 개의 동영상이 있습니다. High school and University-Level Chemistry Help. Ask me questions, I will help you figure it out. 아래는 He2의 MO 이다.[1] 결합성과 반결합성 MO에 모두 전자가 차서 결합차수가 0, 즉 결합을 형성하지 않음을 확인할 수 있으며 이는 계산 결과와 일치한다. MO diagram 만으로는 반결합성 오비탈이... McQuarrie, Physical Chemistry: A Molecular Approach, 1997, University Science Books, p.340 [3]Peter Atkins, Physical Chemistry 11e... This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule. The bond order of He2 is calculated and the meaning of this number ... He2 2+ Molecular Orbital Diagram The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.
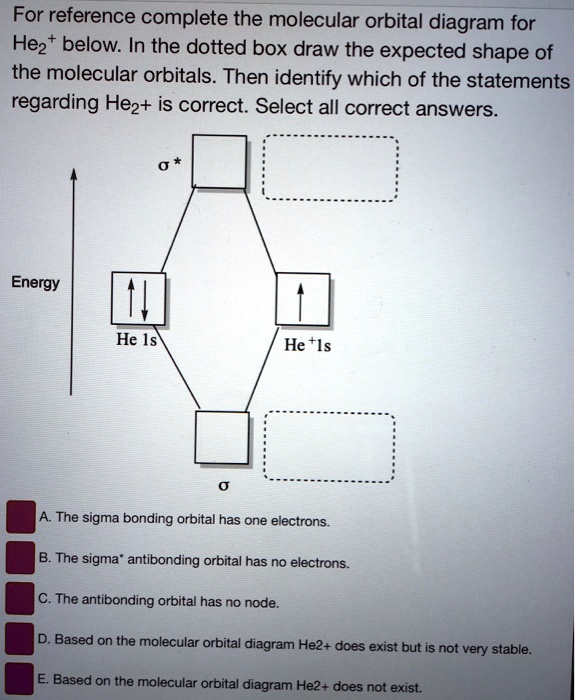
Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order Mar 15, Construct the molecular orbital diagram for He2 + and then identify the bond order. Next Based on molecular orbital theory, the bond order. (1) Sketch an MO energy diagram showing only the molecular orvitals and electiron distribution in CO. Label the energy levels according to the type of orbitals from which they are made, whether they.. How to write simple Molecular Orbital Diagrams and determine the Bond order ※ 원자오비탈(Atomic Orbital) : 주어진 3가지 양자수를 가지는 파동함수인데 이 함수로부터 원자 주위에서 전자를 발견할 확률을 알 수 있다. 원자오비탈은... 통상적인 분자오비탈은 대부분 Linear combination of 원자오비탈들 Molecular Orbirtal (원자오비탈 선형조합-분자오비탈, LCAO-MO법)으로 표시된다 (특히 정성적이거나 아주...
Mar 16, 2019 · Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order. Astak Cm 918t Wiring Diagram; Fj Cruiser Serpentine Belt Diagram; Doosan D80s Forklift Wiring Diagram; Kenwood Kdc 2011s Wiring Diagram; Ezgo Txt 36 Volt Shift Lever Wiring Diagram; Suzuki Eiger 400 Wiring Diagram And Parts; Arduino Uno Dm542t Wiring Diagram The molecular formula is C(2 x 2)H(2 x 3)O(2 x 2)S(2 x 1) = C4H6O4S2 mass S = 0.0550 mol S x 3.118 Let X equal the mass of benzoic acid and Y the mass of gallic acid in the 1.00 g mixture. Therefore, X + Y = 1.00 g. Because both acids contain only one acidic hydrogen, there is a 1 to 1 mol ratio between each acid and NaOH in the acid-base ... Dispersion is therefore absent at the level of Hartree–Fock molecular orbital (MO) theory and has historically been difficult to describe with density functional theory (DFT) as well, because popular semilocal functionals fail to account for long-range electron correlation. Various strategies have been devised to incorporate... ACS ACS Publications C&EN CAS Find my institution Log In ADVERTISEMENT COVID-19 Remote Access Support:Learn... Problem: Construct the molecular orbital diagram for He2 and then identify the bond order. Click within the blue boxes to add electrons.Bond order: a) 0b) 0.5c) 1 d) 1.5e) 2
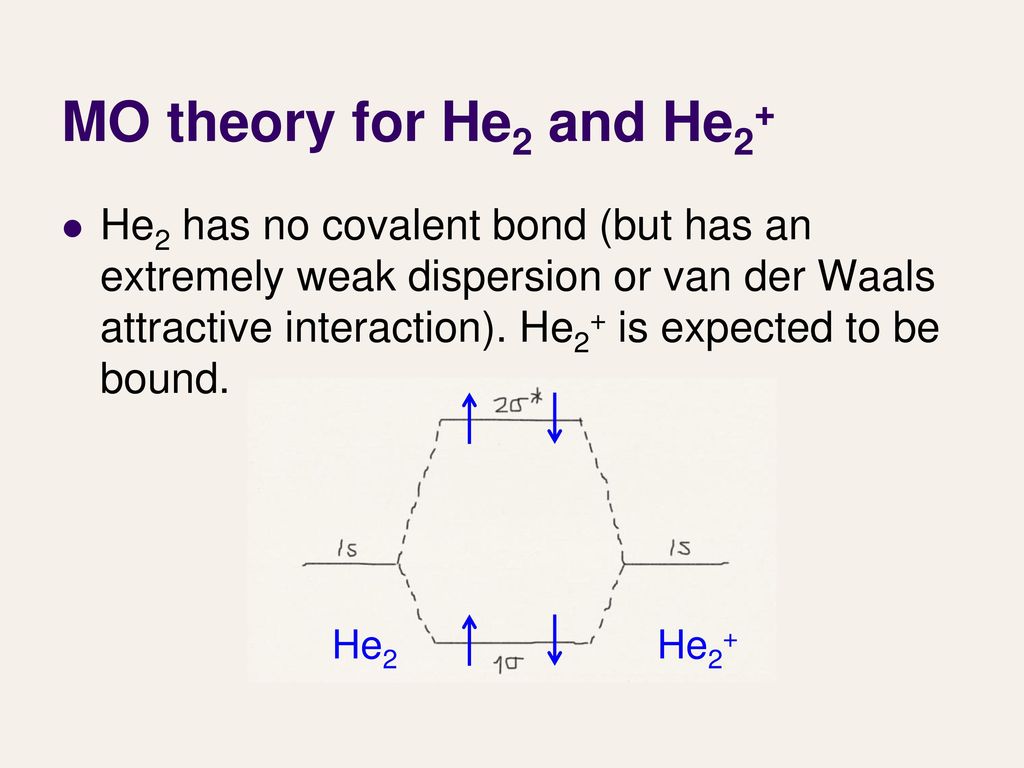
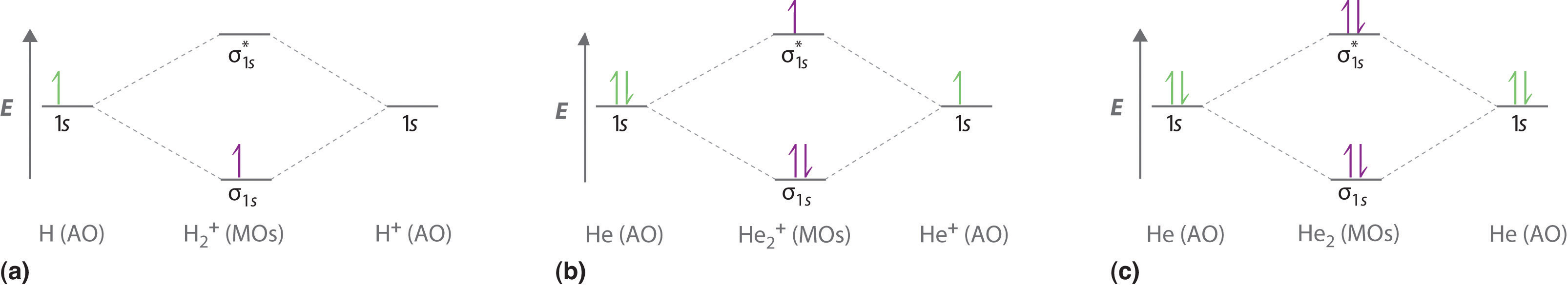
The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2.
The intensity distribution of the photoelectron spectrum was analyzed in terms of rovibronic photoionization selection rules, a simple orbital ionization model... Photoionization and PFI-ZEKE photoelectron spectra of 4 He 2 and 3 He 2 have been recorded and analyzed and a set of ac- curate molecular constants for the lowest...
dimers, -He2+ -Ionic bonding, NaCl -Electronegativity -Ionic crystals -B1 or NaCl -B2 or CsCl -Ionic radii -Size criteria B1 and B2 -Wurtzite or B4 -Sphalerite or B3 -Size... -Woodward-Hoffmann Rules -Orbital correlation diagrams for 2+2 and 2+4 cyloadditions -GVB description H2+D to H+HD exchange reaction 제공기관측의 사정으로...
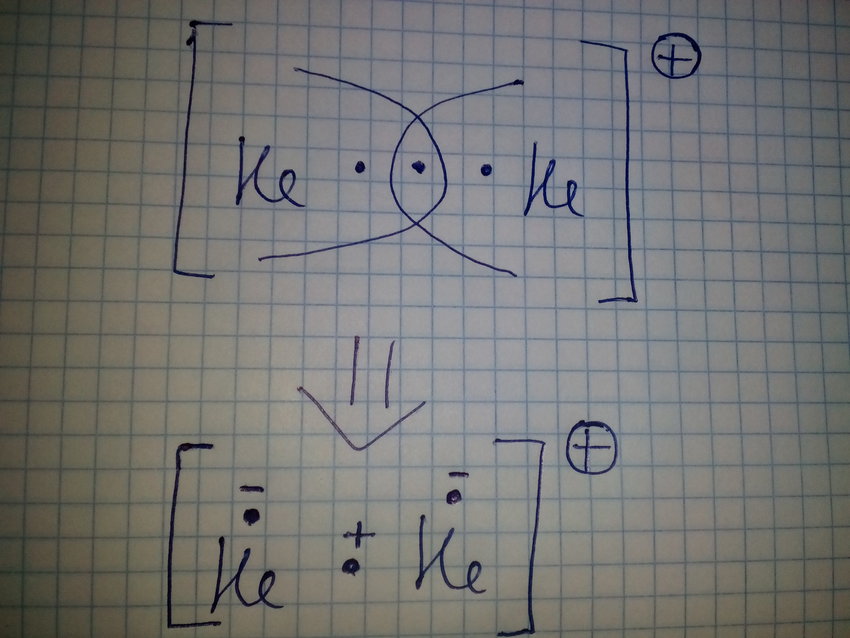
이번 글(1)과 이어지는 다음 글(2)을 통해서는 2주기 원자들이 만든 동핵 이원자 분자의 오비탈(Homonuclear diatomic Molecular Orbital)에 대해 알아보려 한다. 동핵 이원자 분자는 같은 종류의 원자 두 개로 만들어진 분자를 말하며, N , O, F 등이 여기에 해당한다. 1주기 원자인 수소( H)와 헬륨( He)은 1s 오비탈만으로 전자 상태를 나타낼 수 있다. 수소 원자는 1s 오비탈에...
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are...
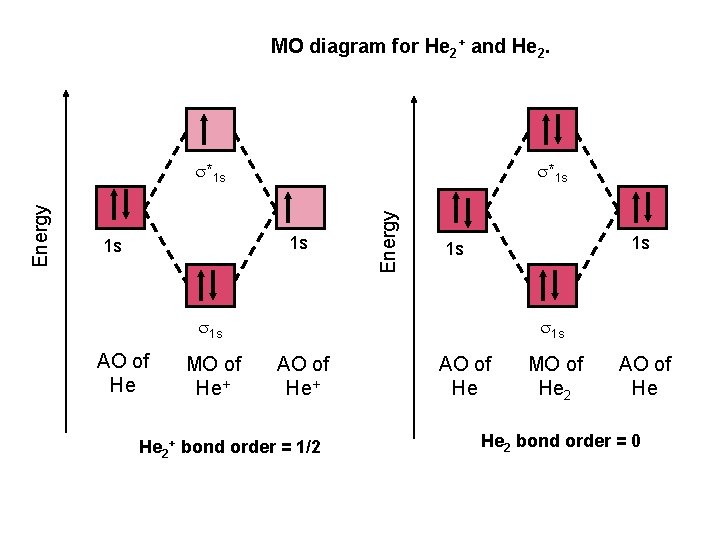
Oct 28, 2021 · Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2.
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule.
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.diatomic …
144 His scientific interests encompass superconductivity, 145 quantum modelling of solids and molecular materials, hydro- and energy storage, hydrogen transfer catalysis, 146 gen 37 applications of high pressures in chemistry, 120 molecular devices, unusual oxidation states of the chemical elements, 147 and more. He now heads...
Transcribed image text: Draw the molecular orbital diagram for Hez Drag the appropriate labels to their respective targets. Reset th Atomic orbital Molecular orbitals Atomic orbital 11 ॥ Antibonding ls ls Energy # He atom Bonding Het ion 1+1 11 He2+ ion. Previous question.
Government strives to have a workforce which reflects gender balance and women candidates are encouraged to apply. EXAMINATION NOTICE NO. 04/2019-CSP DATE :19/02/2019 (LAST DATE FOR RECEIPT OF APPLICATIONS: 18/03/2019) of CIVIL SERVICES EXAMINATION, 2019 (The Commission’s Website: www.upsc.gov.in) IMPORTANT 1. CANDIDATES TO...
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy.
Mulliken, 1896-1986)은 《chemical bond and the electronic structure of moleculaes by the molecular orbital method, 분자의 화학결합 및 전기적 구조에 관한 연구》에 대한 연구 성과를 인정받아 노벨 화학상을 수상했다. 그는 노벨상 수상 강연 《Spectroscopy, molecular orbitals and chemical bonding》에서 다음과 같이 말한다.을 통해 Schrodinger 파동 방정식이 어떻게 분자에...
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. According to the molecular orbital theory, in a supposed He2 molecule, both the bonding and the antibonding orbitals will have 2 electrons each.
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Transcribed image text: Draw the molecular orbital diagram for He, 2+. Drag the appropriate labels to their respective targets. Not all targets will be filled. Reset 1+ Atomic orbital Molecular orbitals Atomic orbital 11 Antibonding ls ls 1 Energy o Het ion Bonding Het ion w 11 Hez2+ ion. Previous question Next question.
Article Open Access Published: 24 November 2021 Unveiling the key factor for the phase reconstruction and exsolved metallic particle distribution in perovskites Hyunmin Kim , Chaesung Lim , Ohhun Kwon , Jinkyung Oh , Matthew T. Curnan , Hu Young Jeong , Sihyuk Choi , Jeong Woo Han & Guntae Kim Nature Communications 12, Article... View all journals Search Login Explore content About the journal Publish with us Sign up for alerts RSS...
Notes Structure of nitrous oxide Keywords nitrous oxide Title Molecular shapes Caption Three-dimensional... Keywords VSEPR, molecular shapes Title Two Charge Clouds Caption Structures of carbon dioxide and hydrogen... Notes Although four charge clouds results in a tetrahedral electronic geometry, the molecular geometry is... orbital, molecular orbital, bonding, antibonding 07-16 Title MO Diagrams for H2 - ion and He2 molecule Caption...
MO - Partial Overlap - Constructive or Destructive Hydrogen 1 and Hydrogen 2 combine to form a new molecular orbital. 결합성 분자오비탈 두 원자핵 사이에 전자가 발견된 확률이 높다. 쉽게 말해서... 한마디로 He2 분자는 존재한 수 없다는 이야기이다. Noble gas 가 왜 안정하고 , 반응하지 않는지 설명을 복잡하게도 햇다. 기본원칙 원자오비탈의 갯수 = 분자 오비탈의 개수... PEET 과수원 과학&수학...
Molecular orbital theory of He2. confusingly showing for the energy diagram for He2 that the change in energy up to the anti-bonding orbital. He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above. Three electrons, two in sigma, one in sigma*.



















0 Response to "38 he2+ molecular orbital diagram"
Post a Comment