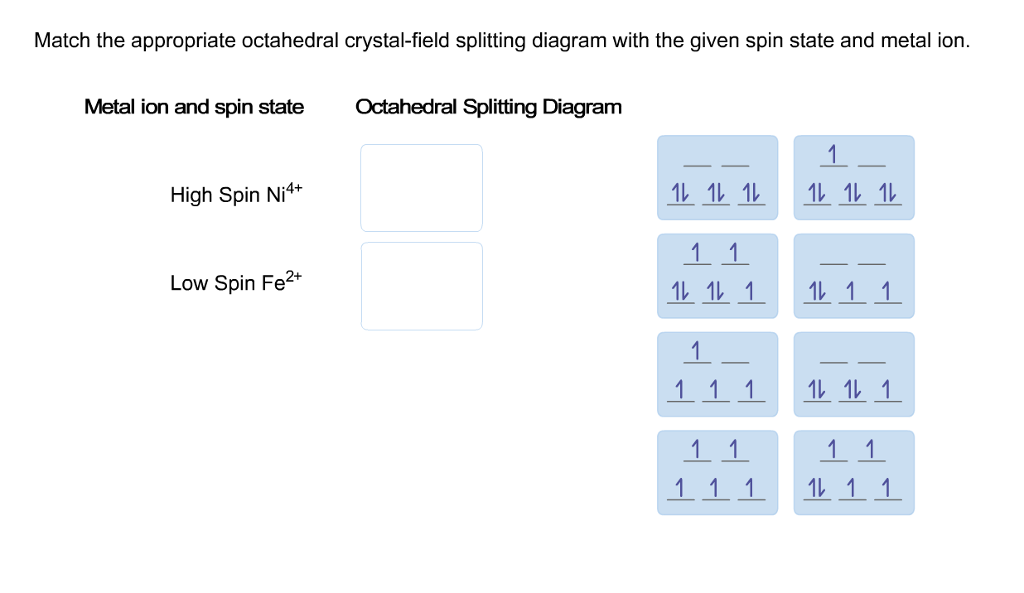
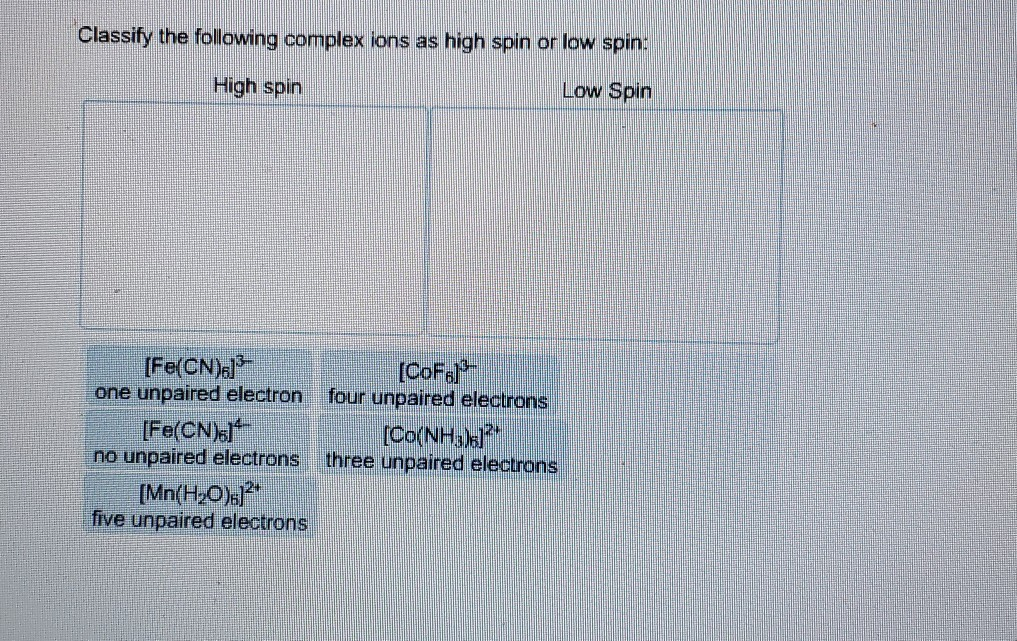
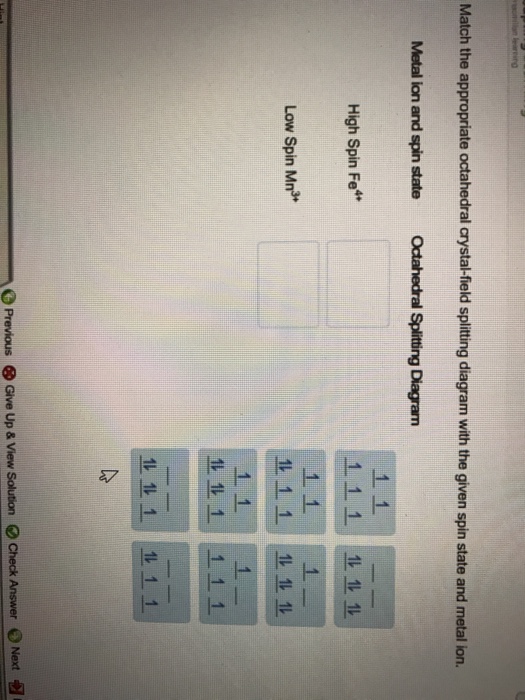
39 match the appropriate octahedral crystal-field splitting diagram fe4+
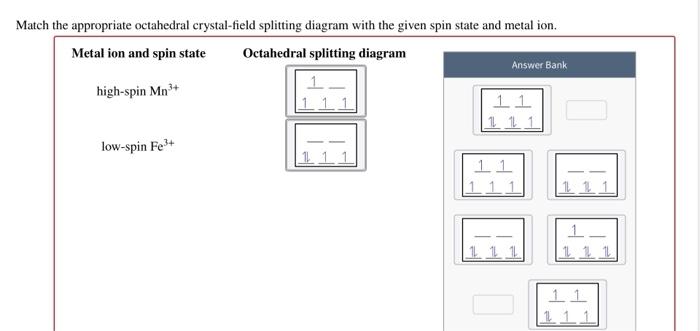
What are the 10 figures of speech? - SolvedLib Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion_ Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4 + 11J low-spin Mn3+ 11 11 1L 1 1 1 1 1 11 1 11... Chapter 5 Skutterudites: Prospective novel thermoelectrics - ScienceDirect Publisher Summary. This chapter describes the relevant physical, chemical, and materials issues pertaining to skutterudites and includes the latest exciting developments. As the search for novel, promising thermoelectrics intensifies; skutterudites have emerged as prospective candidates for achieving figures of merit well in excess of unity.
On the complexity of spinels: Magnetic, electronic ... - ScienceDirect.com Crystal-electric field splitting and the symmetry of 3d orbitals (a) CEF splitting at tetrahedral and octahedral lattice sites. Due to the lattice symmetry and shape of the orbitals, the splitting at the octahedral sites is significantly larger. In tetrahedral symmetry the ground state is labelled e.
Match the appropriate octahedral crystal-field splitting diagram fe4+
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION [PDF ... - AuthorZilla 24 THE CRYSTAL FIELD 13 Other Geometries In 4 coordination, two geometries are common, tetrahedral and square planar, for which the crystal eld splitting patterns are shown in Fig. 1.4. For the same ligand set, the tetrahedral splitting parameter is smaller than that for the octahedral geometry by a factor of 23 because we now have only four ... Electronic Structure of 3d Transition-Atom Impurities in Semiconductors ... S denotes the ground-state spin and J is the (weak-crystal-field) effective total angular momentum into which the substitutional ground-state term is split by spin-orbit interaction in first order. The J > 3/2 of ' E is further split in second order. Calculation of the electronic structure of Y-doped SrTiO3 The magnetic moments and the half-metallicity of X0.75Eu0.25O mainly originate from the spin-polarization of Eu 4f-orbitals, which are caused by the strong octahedral crystal field in the ligand ...
Match the appropriate octahedral crystal-field splitting diagram fe4+. Coordination Compounds [1d47vyp7dy42] Let us explain this splitting in different crystal fields. (a) Crystal field splitting in octahedral coordination entities In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ligands. J.d. Lee Concise Inorganic Chemistry For Jee (main & Advanced) [PDF ... 5.1 Double Salts and Coordination Compounds 5.2 Werner's Work 5.3 More Recent Methods of Studying Complexes 5.4 Classifcation of Ligands 5.5 Effective Atomic Number (EAN) Sidgwick EAN rule 5.6 Shapes of d Orbitals 5.7 Bonding in Transition Metal Complexes Valence bond theory Crystal feld theory Molecular orbital theory 5.8 Valence Bond ... Oswal_Chemistry_Class XII.pdf - Chemistry - Notes - Teachmint Notes of Class 12, Chemistry Oswal_Chemistry_Class XII.pdf - Study Material The Physics and Chemistry of Materials [1. ed.] 0471057940 ... Jahn-Teller Effect Examples of Weak and Strong Crystal Field Effects Crystal Fields and Cr3C in Al2 O3 Experimental Results for in the Free-Spin Limit Spin Glasses and the RKKY Interaction Kondo Effect and s-d Interaction T for Ni ... Square-planar dsp2 and octahedral d2 sp3 hybrid orbitals formed from s, p, and d atomic orbitals on the ...
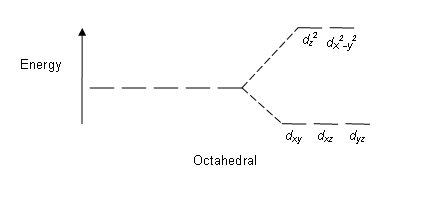
Inorganic Chemistry, 1Ed [1 ed.] 9781259062858 - DOKUMEN.PUB Orgel Diagrams 26.16 Racah Parameters 26.24 Terms Correlation Diagrams under the Effect of Weak and Strong Field Effects 26.26 Tanabe-sugano Diagrams (T-S Diagram) 26.29 Charge-Transfer Transitions 26.34 Types of Magnetism 26.39 Summary 26.55 Solved Examples 26.56 Exercises 26.57 27. The structure of glass: A phase equilibrium diagram approach In an octahedral crystal field it splits to e g and t 2g , where e g is lower level and splits i.e. e g and t 2g are separate into ( 2 B 2g and 2 A 1g ) and ( 2 E g and 2 B 2g ) [41]. Calculation of the electronic structure of Y-doped SrTiO3 The magnetic moments and the half-metallicity of X0.75Eu0.25O mainly originate from the spin-polarization of Eu 4f-orbitals, which are caused by the strong octahedral crystal field in the ligand ... Electronic Structure of 3d Transition-Atom Impurities in Semiconductors ... S denotes the ground-state spin and J is the (weak-crystal-field) effective total angular momentum into which the substitutional ground-state term is split by spin-orbit interaction in first order. The J > 3/2 of ' E is further split in second order.
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION [PDF ... - AuthorZilla 24 THE CRYSTAL FIELD 13 Other Geometries In 4 coordination, two geometries are common, tetrahedral and square planar, for which the crystal eld splitting patterns are shown in Fig. 1.4. For the same ligand set, the tetrahedral splitting parameter is smaller than that for the octahedral geometry by a factor of 23 because we now have only four ...











0 Response to "39 match the appropriate octahedral crystal-field splitting diagram fe4+"
Post a Comment