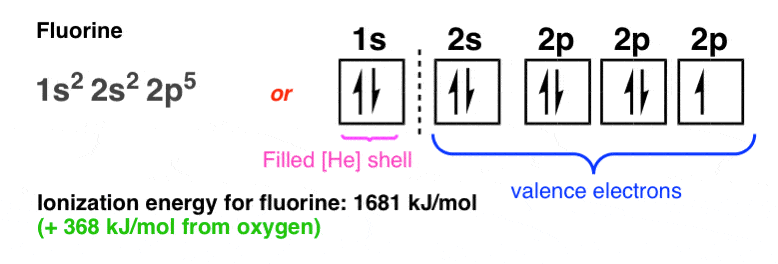
39 write full orbital diagram for f
Lanthanides: Electron Configuration ... - Study.com The f-block elements in the periodic table appear in two series characterized by the filling of the 4f and 5f orbitals. ... an electron enters the orbital with lowest energy first and subsequent ... Valence Electrons Chart for All Elements (Full Chart Inside) Ionization Energy of all Elements (Full Chart Inside) Atomic Radius of All the Elements (Complete Chart Inside) Electron Configuration of All Elements (Full Chart Inside) Protons Neutrons & Electrons of All Elements (List + Images) Orbital Diagram of All Elements (Diagrams given Inside)
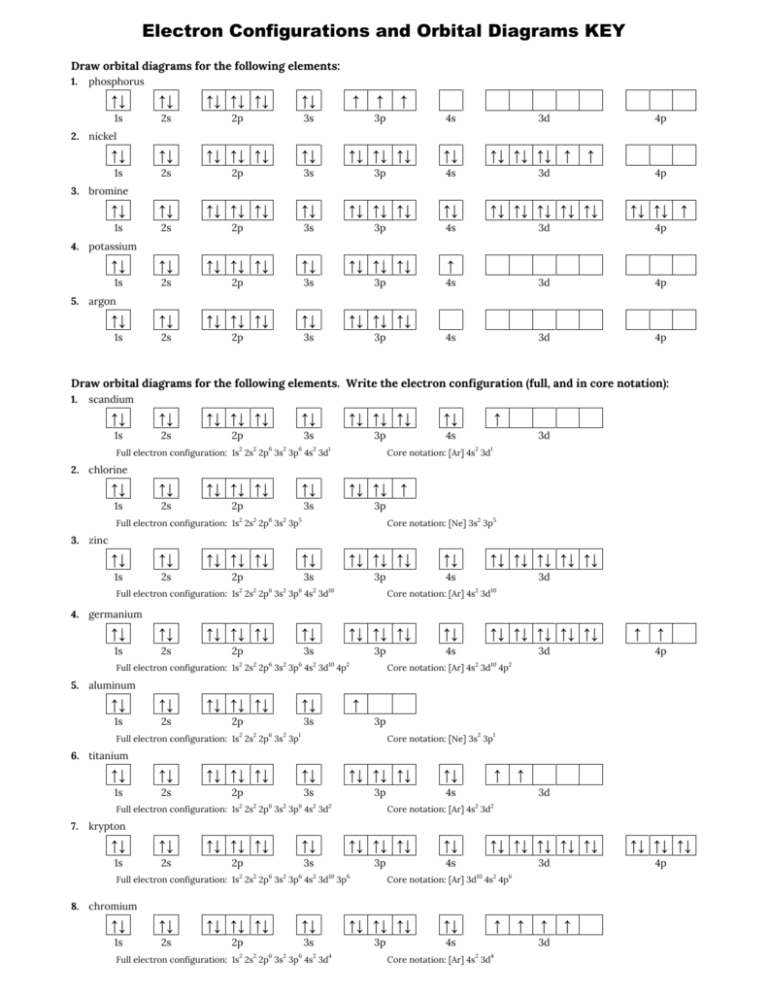
41 orbital diagram for p - Wiring Diagram Images Bromine Orbital Diagram - Wiring Diagrams Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). Note that in linear diatomic molecules, the p_z orbital always points along the internuclear axis, so it has to contribute to one of the sigma bonds.
Write full orbital diagram for f
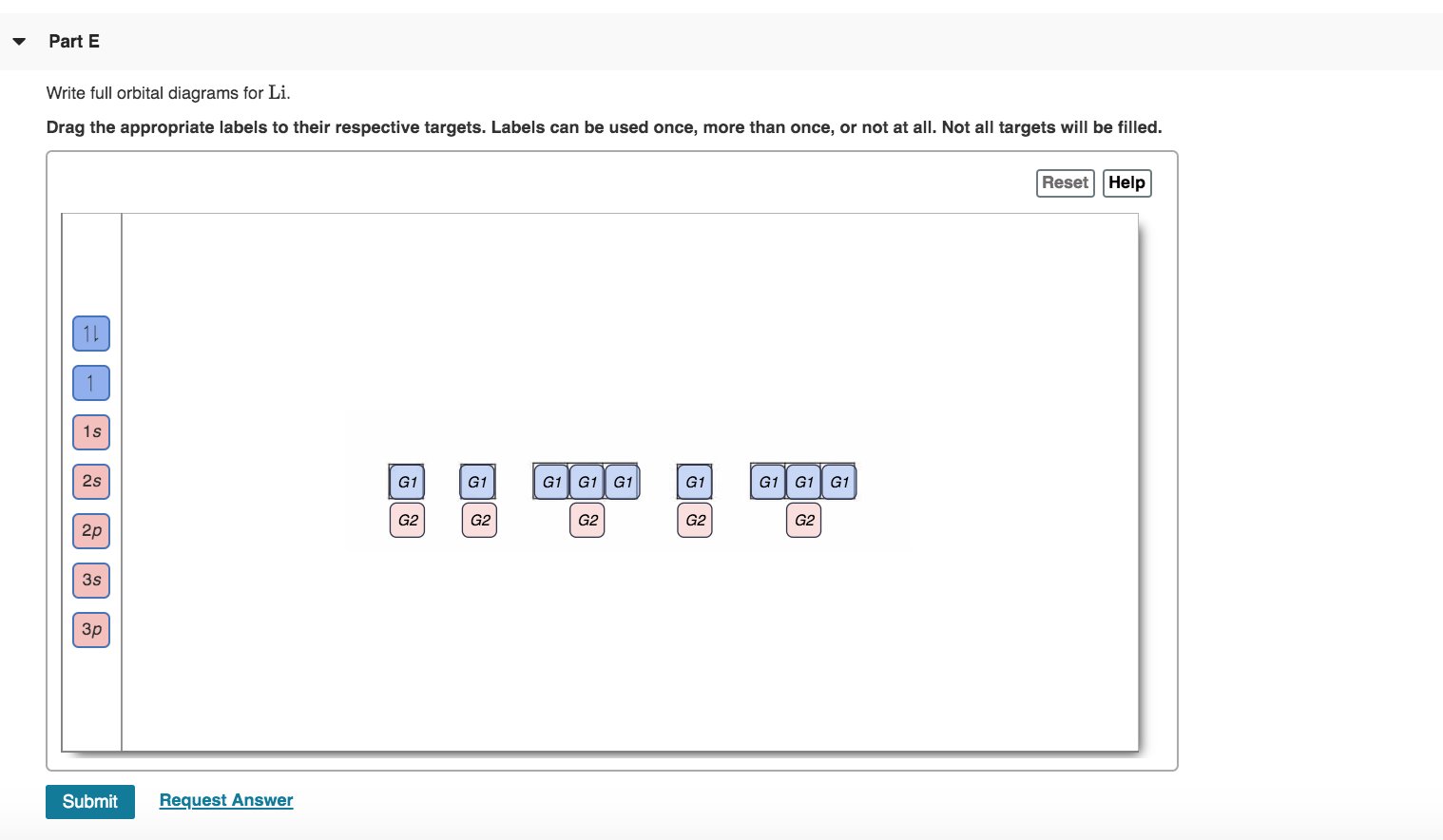
EOF Molecular Orbital Theory: Explanation, Illustrations and ... Molecular orbitals are of two types - bonding and antibonding. The two types of bonds are σ - bond and π − bond. The s -orbitals of one atom can overlap with the s, p, d, f, orbital of another atom such that the overlapped region is symmetrical about the internuclear axis. Similar symmetrical overlaps are also possible among p, d and f ... 44 enter the orbital diagram for the ion mo3+. - Wiring ... 2. Enter the orbital diagram for the ion Au+ 3.Construct the orbital diagram for the ion Mo3+ 4.Construct the orbital diagram for the ion Zr2+ ) Question: 1. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be ...
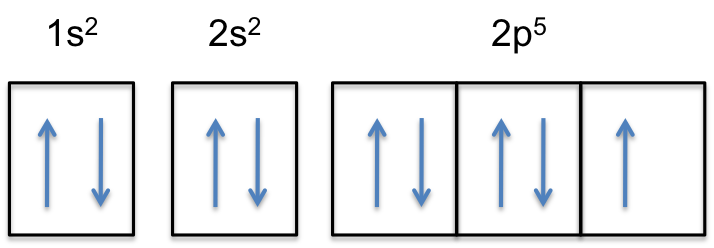
Write full orbital diagram for f. What Are The 3 Rules For Orbital Diagrams Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. This means that when writing orbital diagrams for partially full shells, fill in all of the up-spin electrons before adding any down-spin electrons. … Construct the orbital diagram of the f ion - Soetrust Construct the orbital diagram of the f ion. By soetrust March 29, 2022. Gamers!!! Amazon Luna launches with freebies for Prime subscribers. Amazon Luna special offer for Prime members! How to Write an Electron Configuration - ChemTalk Another way to represent an electron configuration is through an orbital diagram. In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. Orbitals are labeled according to their principle energy levels and sublevels (1s, 2p, etc..). Helium ... 41 electron configuration and orbital filling diagram ... Electron Configuration Worksheet - Easy Hard Science Argon Ar is element 18 with 18 electrons when it's neutral. The 1s orbital is full, the 2s orbital is full, the 2p orbital is full, the 3s orbital is full, and the 3p orbital is full. Fill the orbitals in this order 1s then 2s then 2p then 3s then 3p, from bottom to top on the orbital diagram.
42 fe2+ orbital diagram - Wiring Diagrams Manual 40 orbital diagram of fe - Diagram For You Orbital diagram of fe Atomic Orbital Diagram for Iron (Fe) Iron ion (Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total ... What is the orbital diagram for Helium? - Fata reforms Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. So for the how do the 2s and 3p orbitals differ from the 1s and 2p ... write the full orbital diagram for f select the sketches of a 3d orbital. check all that apply 3p vs 2p orbital 3p orbital shape why are atoms usually portrayed as spheres when most orbitals are not spherically shaped? compare the characteristics of 4d orbitals and 3d orbitals and complete the following sentences. Tellurium electron configuration: Clear your doubt ... An f subshell can have up to 14 electrons in its shell. For writing the electron configuration, we use the Aufbau Principle or which is also called the building up principle. It states the electrons occupy the orbitals in the order of increasing energy.
How To Draw An Orbital Overlap Diagram - Worldanalysis.net Steps for Drawing an Orbital Diagram Label the arrow energy. The arrow shows a qualitative representation of increasing orbital energy. Write out the electron configuration to determine which orbitals are filled. Along the right side of the energy arrow, write each electron shell that is being filled. How To Draw An Orbital Diagram For Magnesium ... How do you write the electron configuration for magnesium? Answer: The first two electrons is placed in the 1s orbital. The next 2 electrons for magnesium go in the 2s orbital. The next six electrons will go in the 2p orbital. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Electron Configuration- Filling Orbitals & Principle Electron Configuration is the distribution of electrons where they are found i.e. Atoms or Ions and one of the best ways to figure out is by looking at the ionization energy. The electrons are arranged in shells, subshells, and orbitals, those on the inside are called core electrons and the ones outside are referred to as valence electrons. Write orbital diagram for Zr2+? Write orbital diagram for Zr2+? July 11, 2021 July 11, 2021 thanh. 3 Answers [Kr]d^2. This is because when ionizing transition state elements, the D and S orbitals are very closely related in energy. We pull the electrons from the S orbital (explaining the exact mechanism would be counter-productive, because it's all hazy theory) rather than ...
Electron Configurations in the s, p & d Orbitals - Video ... Therefore, all the electrons in both hydrogen and helium fit in the first orbital. We write this as 1s^1 for hydrogen, since it is in the first s orbital and has one electron. Meanwhile, we write ...
Orbital Diagrams And Electron Configuration Worksheet Answers Full electron configuration of uranium. Write a gold state electron configuration for each neutral atom. Do quiz and orbital diagram notation use quizizz. Electron configuration practice keypdf. Which brand is invalid character in determining factor in print and orbital diagrams electron configuration worksheet answers with a great quiz, turn ...
44 enter the orbital diagram for the ion mo3+. - Wiring ... 2. Enter the orbital diagram for the ion Au+ 3.Construct the orbital diagram for the ion Mo3+ 4.Construct the orbital diagram for the ion Zr2+ ) Question: 1. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be ...
Molecular Orbital Theory: Explanation, Illustrations and ... Molecular orbitals are of two types - bonding and antibonding. The two types of bonds are σ - bond and π − bond. The s -orbitals of one atom can overlap with the s, p, d, f, orbital of another atom such that the overlapped region is symmetrical about the internuclear axis. Similar symmetrical overlaps are also possible among p, d and f ...
EOF



















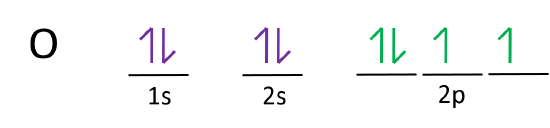







0 Response to "39 write full orbital diagram for f"
Post a Comment